Nanozyme for Tumor Catalytic Therapy: A New Biomimetic Strategy
Researchers from the Institute of Biophysics, Chinese Academy of Sciences, led by Prof. YAN Xiyun and Prof. FAN Kelong, have made new progress in the study of tumor catalytic therapy using ultrasmall metal alloy nanozymes mimicking neutrophil enzymatic cascades. The related paper was published in Nature Communications.
Nanozymes are a new type of catalyst that can catalyze enzyme substrates under physiological or low-temperature, high-temperature conditions, serving as substitutes for natural enzymes for human health. In the field of tumor treatment, the strategy of nanozymes catalyzing hydrogen peroxide (H2O2) to generate reactive oxygen species for killing tumor cells has great potential. However, the low concentration of H2O2 (less than 0.1 mM) in the tumor microenvironment limits its therapeutic effectiveness. The neutrophil superoxide dismutase (SOD) and myeloperoxidase (MPO) cascade killing mechanism provides a new approach to overcoming this issue. In this mechanism, H2O2 is produced from SOD and serves as a substrate for MPO. However, there are few reports of nanozymes with MPO-like activity, and developing nanozymes that possess both SOD- and MPO-like activities is even more challenging.
The researchers develop nanozymes possessing both SOD- and MPO-like activities through alloying Au and Pd, which exhibits the highest cascade activity when the ratio of Au and Pd is 1:3, attributing to the high d-band center and adsorption energy for superoxide anions. Therefore, the Au1Pd3 alloy nanozyme can mimic the SOD-MPO cascade killing effect of neutrophils by generating HClO and 1O2 to cause DNA damage and cell apoptosis. This nanozyme significantly inhibited tumor growth in mouse colon cancer CT26 and breast cancer 4T1 xenograft models, leading to a noticeable extension of the survival period of tumor-bearing mice.
Additionally, the Au1Pd3 alloy nanozyme exhibited good in vivo safety, mainly due to the high concentration of its catalytic substrate O2·- in tumor cells compared to normal cells, giving it tumor-specific cytotoxicity. The ultra-small size of this nanozyme (less than 6 nm) endowed it with renal clearance function, avoiding long-term accumulation in the body.
This study's biomimetic strategy of simulating neutrophil multi-enzyme cascade reactions for tumor treatment will drive the development of more biomimetic treatment methods for use in antibacterial, antitumor, or other disease treatments.
Furthermore, the approach of using multi-enzyme activity nanozymes to mimic various natural enzymes in phagosomes will also promote research on nanozyme-mimicking organelles (such as lysosomes, peroxisomes, etc.). Additionally, the MPO-like activity possessed by the nanozyme in this study is a novel type of nanozyme catalysis, with limited current research. Given its potential applications in tumor treatment, antibacterial, and other fields, as well as the elucidation of its catalytic mechanism in this study, more discoveries and designs of MPO-like activity nanozymes are likely to be promoted.
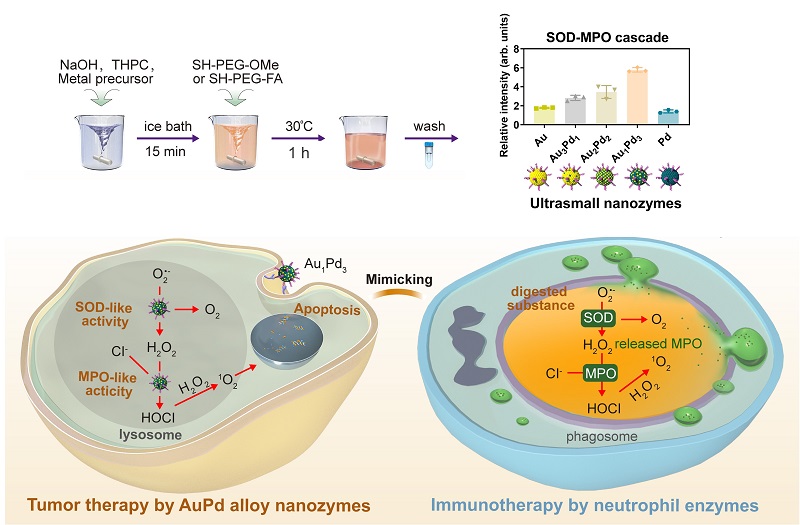
Fig. Development of Neutrophil Enzyme-Induced Nanozymes and Their Application in Tumor Catalytic Therapy
Article link: https://www.nature.com/articles/s41467-024-45668-3
Contact: FAN Kelong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Email: fankelong@ibp.ac.cn
(Reported by Prof. FAN Kelong's group)

