Phase Separation by Fusion Protein FC Reveals New Mechanism for Tumor Development in Rare Cancer
The dynamic remodeling of the endomembrane system is a core biological process essential for maintaining cellular compartmentalization and homeostasis. Proteins enriched in intrinsically disordered regions (IDRs) can drive membrane curvature formation in artificial vesicles through liquid-liquid phase separation (LLPS); however, direct evidence for LLPS-mediated endomembrane remodeling in living cells has been lacking.
On April 23, 2025, Prof. LI Dong's group from the Institute of Biophysics, Chinese Academy of Sciences, and the School of Life Sciences at Tsinghua University, using Multi-SIM super-resolution imaging technology, provided direct evidence in living cells that transmembrane fusion proteins (MFPs) rich in IDRs can remodel targeted endomembrane systems via LLPS. This study was published in Molecular Cell.
Using the characteristic fusion protein FUS-CREB3L2 (FC) from low-grade fibromyxoid sarcoma (LGFMS) as a model, the study systematically elucidated the molecular mechanism by which aberrant phase separation drives membrane remodeling and promotes tumorigenesis, offering a new perspective for understanding the biological functions of interactions between LLPS and endomembrane systems.
The FC protein retains both the IDRs from FUS and the transmembrane domain and DNA-binding domain from CREB3L2. By constructing an inducible FC expression system, the researchers utilized Multi-SIM imaging to capture over 2,300 time points across six continuous hours, recording for the first time the dynamic process by which FC remodels the endoplasmic reticulum (ER) through LLPS.
The researchers discovered that the remodeled ER membrane structures co-localized with COPII vesicle markers, forming COPII-like compartments with diameters significantly larger than those of classic COPII vesicles. These compartments sequestered the proteases S1P/S2P, triggering spontaneous intramembrane proteolysis of FC, thereby releasing its transcriptionally active N-terminal fragment (FC-N) into the nucleus.
In contrast, the wild-type CREB3L2 protein localizes to the ER without altering ER membrane morphology, and its nuclear translocation requires stimulation with the ER stress inducer brefeldin A (BFA) to undergo "induced" cleavage at the Golgi apparatus.
Inside the nucleus, FC-N specifically recruits SSRP1 and CHD7 to form a transcriptional complex, activating the expression of LGFMS-characteristic genes and ultimately inducing malignant cellular phenotypes.
This study is the first to reveal the regulatory pathway by which FC-driven aberrant phase separation leads to ER membrane remodeling and subsequent nuclear signaling, providing a crucial theoretical basis for the development of targeted therapies against LGFMS.
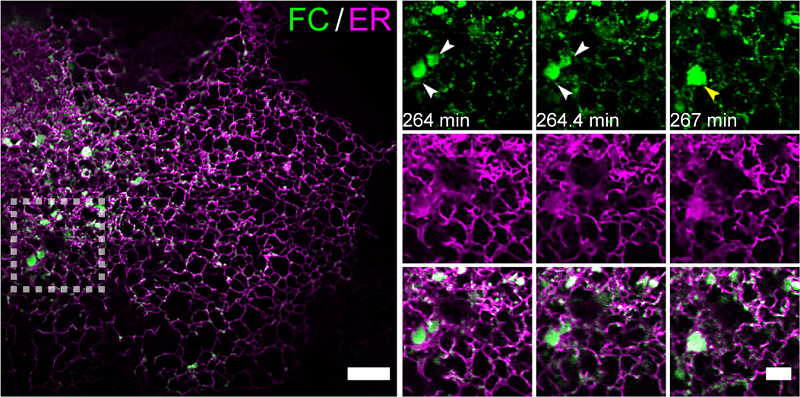
Figure 1. Multi-SIM imaging revealed the accumulation and condensation effects of FC on the endoplasmic reticulum membrane
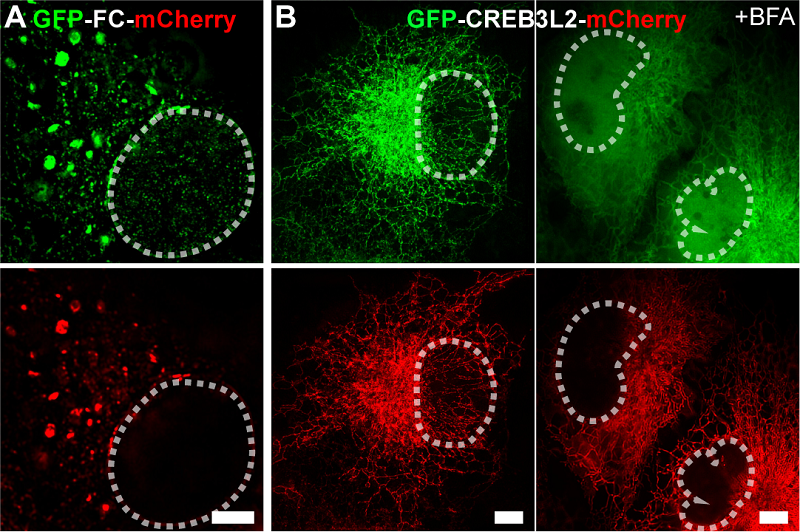
Figure 2. Multi-SIM images of GFP-FC-mCherry fusion protein (A) and GFP-CREB3L2-mCherry (B). Scale bar: 5 µm
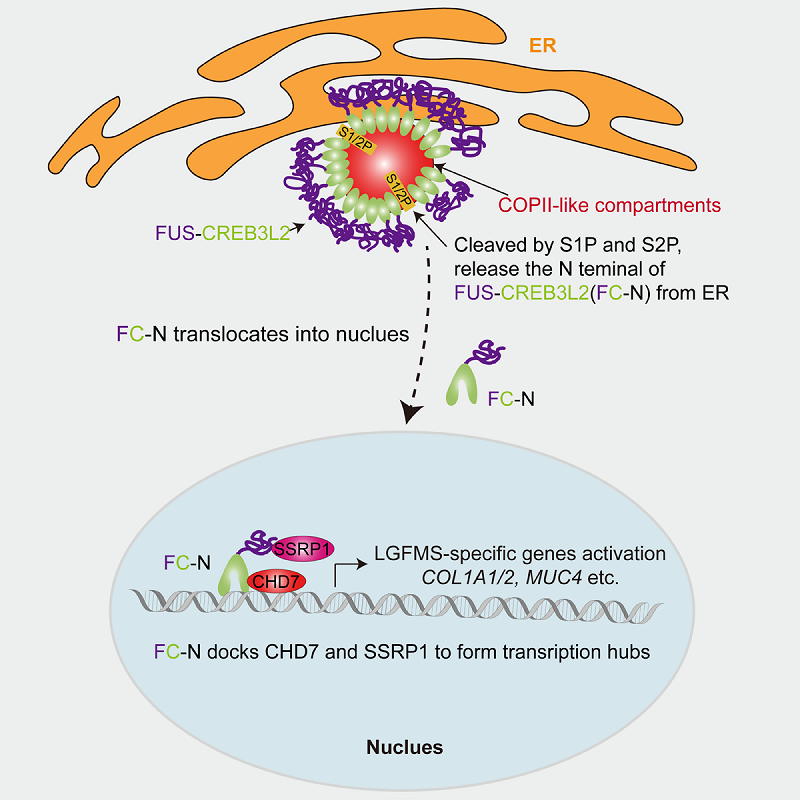
Figure 3. Schematic diagram of the oncogenic mechanism of FC
(Image by LI Dong's group)
Article link: https://doi.org/10.1016/j.molcel.2025.04.001
Contact: LI Dong
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: lidong@ibp.ac.cn
(Reported by Prof. LI Dong's group)

