Structural Insights Reveal How the Kinesin-2 Motor Complex Assembles for Intraflagellar Transport
Within living organisms, each cell operates as a highly organized and dynamic "miniature city." Kinesins serve as essential "couriers" in this city, transporting various intracellular "packages"-including proteins, organelles, and vesicles-along the cell's internal "highways," known as microtubules. These motor proteins ensure that cargo is delivered precisely to where it's needed, maintaining the efficiency and function of the cellular system.
Kinesin-2 is a specialized subclass of kinesins. The canonical kinesin-2 forms a stable heterotrimeric complex composed of two distinct motor subunits (e.g., KIF3A/KIF3B or KIF3A/KIF3C) and one non-motor subunit, KAP3. This heteromeric configuration is essential for specialized processes such as anterograde transport in cilia. Despite its importance, the molecular mechanisms governing the assembly of this complex remain poorly understood.
On July 24, 2025, a research team led by Prof. FENG Wei at the Institute of Biophysics of the Chinese Academy of Sciences, published a study in Nature Communications shedding light on the structural and functional basis of kinesin-2 assembly. Focusing on the heterotrimeric kinesin-2 complex from Caenorhabditis elegans-comprising KLP-11, KLP-20, and KAP-1-the team combined structural biology with in vivo functional analyses to elucidate the mutual co-recognition mechanisms underlying complex formation and its physiological relevance.
The researchers first reconstituted the heterotrimeric kinesin-2 complex by co-expressing its components in vitro and determined its overall architecture using single-particle cryo-electron microscopy. This structural framework guided further protein optimization, culminating in a 3.5 Å resolution crystal structure resolved through X-ray crystallography.
The structure revealed that the tails of the two motor subunits intertwine via conserved hetero-pairing trigger sequences (HTSs), forming a heterodimer that cooperatively engages the C-terminal helical hook (CTH-hook) of KAP-1. Simultaneously, the central target-binding groove of KAP-1 anchors both motor tails, stabilizing the entire complex. This arrangement illustrates a precise and robust mode of assembly based on mutual co-recognition among the three subunits.
To assess the functional relevance of these structural features, the team introduced targeted mutations into key interface residues and examined their impact on complex formation using co-immunoprecipitation assays. These experiments confirmed that the identified interfaces are critical for kinesin-2 assembly.
The study then extended to live C. elegans, where the researchers expressed the mutants in sensory neuron cilia and visualized intraflagellar transport (IFT) dynamics using fluorescently labeled IFT proteins. These in vivo analyses further validated that mutual co-recognition is essential for efficient ciliary transport.
Interestingly, the interface regions of the kinesin-2 complex contain multiple putative phosphorylation sites. Phosphorylation at these positions may destabilize the interaction interfaces and promote complex dissociation, suggesting a regulatory mechanism that allows dynamic cycling of the kinesin-2 complex during ciliary transport.
"Mutations in genes such as KIF3A and KIF3B are associated with a range of ciliopathies, including retinitis pigmentosa, polycystic kidney disease, and neurodevelopmental disorders," Prof. FENG noted. "Understanding how kinesin-2 assembles not only offers insights into the molecular basis of these diseases but also opens potential avenues for targeted therapies and gene-based interventions."
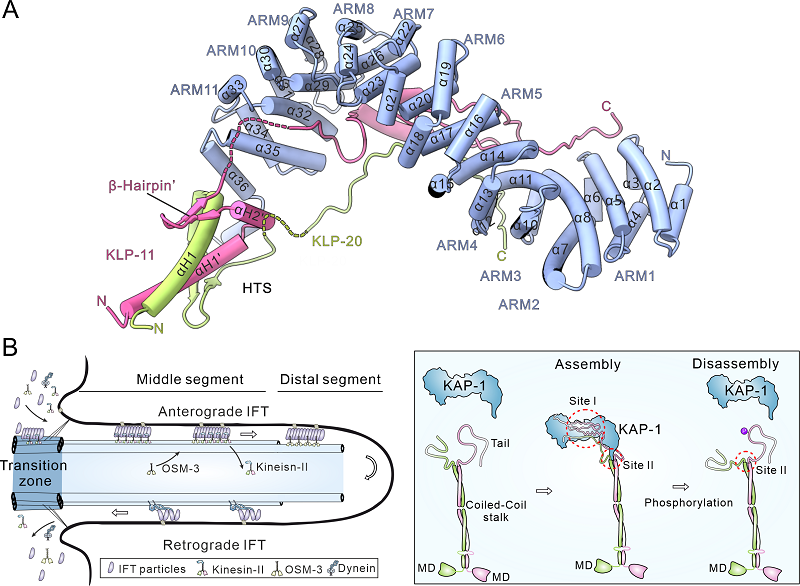
Figure. Overall structure of the tail region of the heterotrimeric kinesin-2 motor and schematic model of its role in regulating intraflagellar transport (IFT)
(Image by FENG Wei's group)
Article link: https://doi.org/10.1038/s41467-025-62152-8
Contact: FENG Wei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: wfeng@ibp.ac.cn
(Reported by Prof. FENG Wei's group)

