IRE1α Hyperactivation by Manganese Offers New Cancer Strategy
IRE1α (inositol-requiring enzyme 1α) is one of the most conserved signaling branches of the unfolded protein response (UPR). While it helps maintain endoplasmic reticulum (ER) homeostasis under stress, sustained or excessive activation of IRE1α under severe or chronic ER stress can lead to cell death via downstream pathways such as regulated IRE1α-dependent decay (RIDD) and JNK signaling. Whether artificially enhancing IRE1α activation in cancer cells could suppress tumor growth has long been an open question - and one with limited exploration to date.
Recently, a research team led by Prof. WANG Likun from the Institute of Biophysics of the Chinese Academy of Sciences, has identified divalent manganese ions (Mn²⁺) as a potent and selective activator of IRE1α. This study, published in iScience on July 15, 2025, reveals that Mn²⁺ can hyperactivate IRE1α, initiate pro-apoptotic signaling, and ultimately suppress tumor growth - offering a promising new direction in cancer therapy.
The researchers demonstrated that Mn²⁺ significantly enhances IRE1α phosphorylation and oligomerization under ER stress conditions, thereby activating pro-death pathways such as RIDD and JNK and inducing apoptosis in breast cancer cells.
Mechanistic studies further showed that Mn²⁺ directly binds to the cytosolic domain of IRE1α, stabilizing the protein and boosting both its kinase and RNase activity. This provides a molecular foundation for its pro-apoptotic effect.
In vivo, intratumoral injection of Mn²⁺ markedly suppressed tumor growth in an orthotopic breast cancer model. Crucially, this anti-tumor effect was completely abolished in IRE1α-deficient tumors, confirming that the therapeutic benefit is strictly IRE1α-dependent.
Following Mn²⁺ treatment, tumor tissues showed significantly elevated levels of JNK phosphorylation and apoptosis, while treated mice exhibited no significant weight loss or adverse effects, suggesting a favorable safety profile in the short term.
This study is the first to propose a "pro-activation" approach to targeting IRE1α for cancer therapy, rather than the conventional strategy of inhibition. The approach may be particularly effective in tumors with intrinsically high IRE1α activity and opens up new possibilities for harnessing the UPR signaling network in oncology.
Given that Mn²⁺ compounds are already used clinically - for example, as MRI contrast agents - these findings support the potential repurposing of Mn²⁺ as a tumor-targeting therapeutic agent. With further refinement, Mn²⁺-based therapies could become a new class of anti-cancer treatments aimed at selectively triggering cell death in tumors via IRE1α hyperactivation.
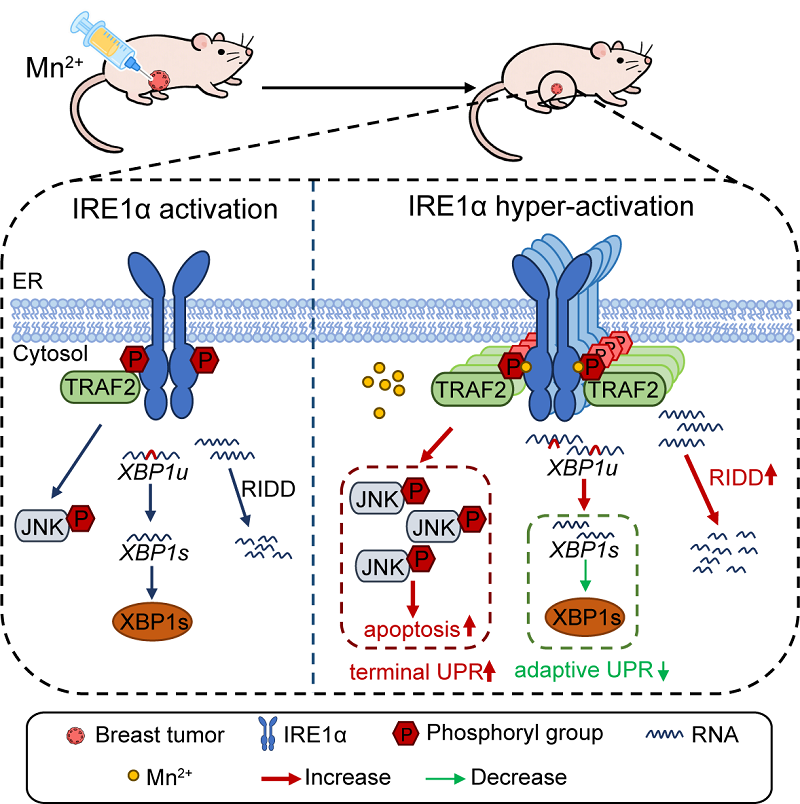
Figure: Schematic illustration of the mechanism by which manganese hyperactivates IRE1α to suppress tumor growth
(Image by WANG Likun's group)
Article link: https://www.cell.com/iscience/fulltext/S2589-0042(25)01382-3
Contact: WANG Likun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: wanglikun@ibp.ac.cn
(Reported by Prof. WANG Likun's group)

