S-nitros(yl)ation of CaMKIIα and its precision redox regulation by SNOTAC plays a critical role in learning and memory
On August 6, 2025, a study led by Prof. CHEN Chang at the Institute of Biophysics of the Chinese Academy of Sciences, in collaboration with Prof. HUANG Zhangjian at China Pharmaceutical University, published in Redox Biology, discovered that S-nitros(yl)ation of CaMKIIα-a redox-based post-translational modification-is a previously unknown molecular mechanism essential for learning and memory. And researchers developed SNOTAC, a precision S-nitrosation modulator distinct from conventional nitric oxide (NO) donors, offering a promising new therapeutic strategy for memory impairment.
Previous work by the Chen group found that in the hippocampus of elderly humans and aged mice, the protein level of S-nitrosoglutathione reductase (GSNOR) significantly increased with age. Concurrently, the level of S-nitrosation - a redox-dependent post-translational modification where NO or its derivatives convert protein cysteine thiols (-SH) to S-nitrosothiols (-SNO) - on CaMKIIα (Ca2+/calmodulin-dependent protein kinase IIα), a protein critical for learning and memory in the hippocampus, significantly decreased. These findings suggested that decreased S-nitrosation of CaMKIIα might be associated with age-related learning and memory impairment. However, whether there was a direct causal link and whether this modification itself played a functional role in learning and memory remained unclear.
To address these questions, researchers first investigated whether the level of CaMKIIα S-nitrosation changes during physiological learning and memory processes. They discovered that after wild-type mice performed learning and memory tasks, the level of CaMKIIα S-nitrosation in their hippocampus significantly increased. This was the first time a dynamic change in the S-nitrosation level of a specific protein was detected during physiological learning and memory tasks.
To directly verify the function of CaMKIIα S-nitrosation, researchers generated a mutant mouse model incapable of undergoing this modification. Across multiple behavioral tests, these mutants displayed pronounced deficits in learning and memory. These results collectively confirmed that S-nitrosation of CaMKIIα is a novel mechanism regulating physiological learning and memory processes.
Mechanistic studies revealed that the loss of CaMKIIα S-nitrosation led to an abnormal increase in the release of synaptic vesicles during the resting state. Researchers vividly termed this the "leaky" effect - analogous to electrical leakage causing wasteful energy consumption. The consequence was that when a requiring response for learning and memory tasks occurred, the synapse's signaling response capacity was significantly insufficient, ultimately leading to impaired learning and memory function.
Having established the core regulatory role of CaMKIIα S-nitrosation in memory, researchers further explored whether precisely increasing this modification level could rescue memory impairment. Traditional methods of regulating S-nitrosation (modulating NO levels) are often non-specifically affecting numerous proteins and potentially causing off-target effects.
Based on the "precision redox" concept proposed by Prof. Chen Chang and the "5R" principles (Right time/place/level/target/species) for antioxidant drug development, researchers pursued precision intervention strategies targeting CaMKIIα S-nitrosation.
For the precise regulation of CaMKIIα S-nitrosation, researchers proposed another universal "glue" strategy - using a small molecule to bring the target protein close to nitric oxide synthase (NOS), allowing the locally produced NO to selectively modify the target protein, thus achieving precise S-nitrosation. Based on this, they innovatively designed and synthesized a S-nitrosation Targeting Chimera (SNOTAC).
SNOTAC specifically brings neuronal nitric oxide synthase (nNOS) close to the target protein CaMKIIα, thereby precisely and selectively enhancing its S-nitrosation. Intranasal administration of SNOTAC effectively rescued memory deficits in mice exhibiting learning and memory impairments due to brain-specific overexpression of GSNOR (mimicking age-related decline in S-nitrosation levels).
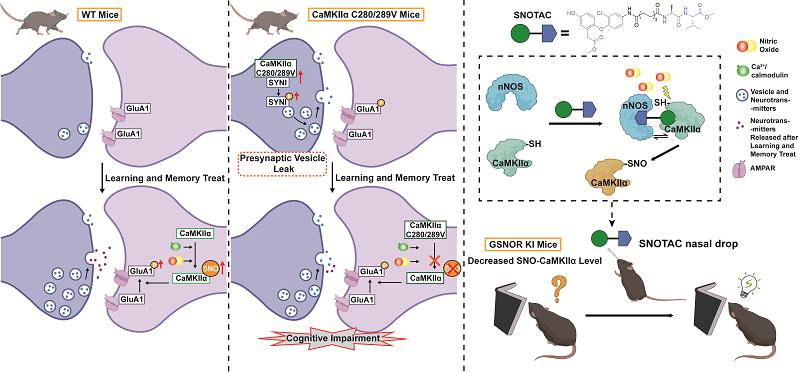
Figure. CaMKIIα S-nitrosation and its precise regulation play a key role in learning and memory
(Image by CHEN Chang's group)
Article link: https://doi.org/10.1016/j.redox.2025.103784
Contact: CHEN Chang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: changchen@ibp.ac.cn
(Reported by Prof. CHEN Chang's group)

