Scientists Reveal How a Human Retrotransposon Enzyme Recognizes DNA Structures to Reshape the Genome
Long Interspersed Nuclear Element-1 (LINE-1 or L1) is the only autonomously active retrotransposon in the human genome and serves as the primary vehicle for the mobilization of most other retrotransposons. Its retrotransposition process is mediated by the reverse transcriptase ORF2p, through a mechanism known as target-primed reverse transcription (TPRT). However, the molecular details governing how LINE-1 targets and integrates into genomic DNA have remained elusive until now.
October 9, 2025 - A collaborative team led by Profs. XU Ruiming, ZHU Bing, and XUE Yuanchao from the Institute of Biophysics of the Chinese Academy of Sciences, published a research article in Science, unveiling the molecular mechanisms that underlie several core functions of LINE-1 retrotransposition.
The researchers successfully purified an active ORF2p-endogenous nucleic acid complex and resolved its high-resolution three-dimensional structure using single-particle cryo-electron microscopy (cryo-EM). The structural analysis revealed that ORF2p binds genomic DNA through strong surface electrostatic interactions, and that cDNA synthesis occurs at the reverse transcriptase (RT) active center through allosteric modulation of multiple amino acid residues.
They further discovered that ORF2p exhibits high catalytic activity toward specific forked DNA substrates, particularly flap structures characteristic of the lagging strand during DNA replication. Conformational changes in the endonuclease (EN) domain enable stepwise cleavage of double-stranded DNA.
Cross-species structural comparisons with homologous proteins from bacteria and insects revealed both conserved and divergent features in retrotransposase evolution: while the reverse transcription activity is highly conserved, the mechanisms for DNA recognition and cleavage have undergone significant diversification.
Importantly, the researchers established the first efficient in vitro DNA cleavage system for ORF2p, demonstrating that it functions as a structure-dependent endonuclease. This finding provides a molecular basis for the coupling between LINE-1 integration and the cell replication cycle.
both structural and biochemical perspectives, this study elucidates the nucleic acid binding mode of ORF2p and the DNA structure-mediated mechanism governing its site-specific cleavage during LINE-1 retrotransposition. ORF2p interacts primarily with the DNA backbone through electrostatic forces, rather than sequence-specific recognition, highlighting its unique physiological role.
This work fundamentally advances the understanding of the mechanism of LINE-1 retrotransposition and provides a new theoretical framework for developing therapeutics targeting retrotransposon regulation.
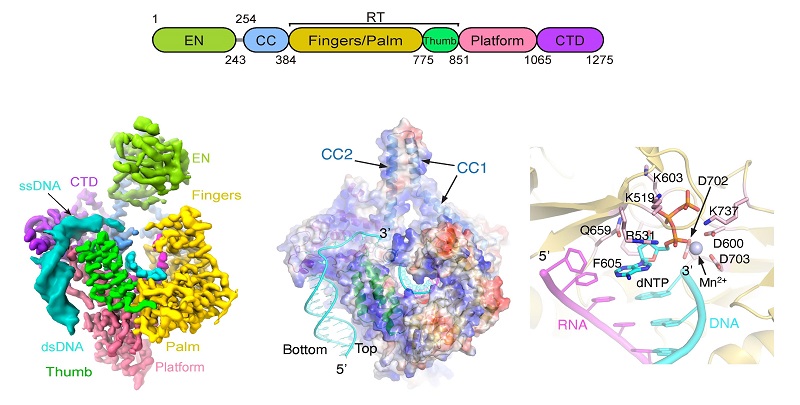
Figure 1. Structure of the functional LINE-1 ORF2p retrotransposition complex
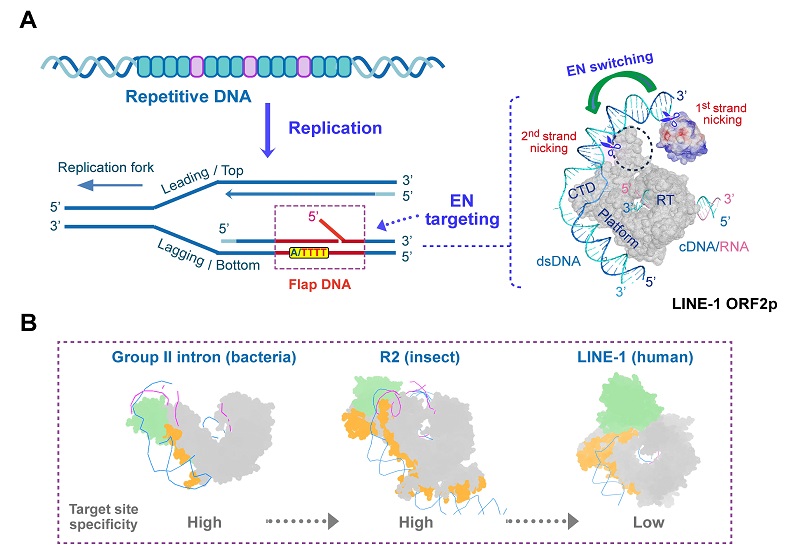
Figure 2. Molecular mechanism of DNA targeting and cleavage by LINE-1 reverse transcriptase. (A) LINE-1 specifically recognizes fork-like DNA structures formed during replication and achieves double-stranded DNA cleavage through conformational changes in the EN domain. (B) Structural comparison of reverse transcriptases from lower to higher organisms.
(Image by XU Ruiming's group)
Article link: https://www.science.org/doi/10.1126/science.adu3433
Contact: XU Ruiming
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: rmxu@ibp.ac.cn
(Reported by Prof. XU Ruiming's group)

