Structural Study Reveals CFAP77 Complex Critical for Sperm Tail Integrity
The core structure of cilia and flagella-the axoneme-is composed of microtubules and associated proteins arranged into a precise and stable architecture. The outer region of the axoneme consists of nine doublet microtubules (DMTs), each formed by a complete A-tubule and an incomplete B-tubule. Identifying the molecular components and regulatory mechanisms that link the A- and B-tubules within DMTs has long been one of the central questions in cilia research, holding key significance for understanding axonemal assembly, ciliary motility, and the pathogenesis of ciliopathies.
On October 21, 2025, a collaborative study from the Institute of Biophysics of the Chinese Academy of Sciences, and Beijing Normal University was published in PLOS Biology. The research addresses a long-standing question in the field: How are the A- and B-tubules of axonemal DMTs stably connected in cilia and flagella?
By combining gene knockout mouse models with in situ structural biology techniques, the researchers identified CFAP77 as a core outer junction (OJ) protein that mediates the connection between A- and B-tubules in axonemal DMTs. They further uncovered the molecular cascade triggered by the loss of CFAP77-namely, the disruption of the CFAP77-CCDC105-TEX43 ternary complex, structural breakage of the A-B connection at the OJ site, and consequent impairment of ciliary and flagellar motility.
Using a Cfap77 knockout (Cfap77-KO) mouse model, the team observed that male mice were infertile, exhibiting normal sperm count and morphology but markedly reduced sperm motility. Transmission electron microscopy revealed that approximately 45% of the DMT B-tubules in Cfap77-KO sperm axonemes were open at the OJ regions. This precise correspondence between protein loss and structural phenotype confirmed the critical role of CFAP77 in maintaining the OJ linkage between A- and B-tubules.
Further cryo-electron tomography (cryo-ET) combined with AI-assisted analysis allowed the researchers to resolve the in situ axonemal structure of Cfap77-KO sperm in unprecedented detail. The results showed that deletion of CFAP77 led to the complete loss of the CFAP77-CCDC105-TEX43 ternary complex at the OJ regions, resulting in open B-tubules and defective sperm motility.
This study demonstrates the feasibility and power of integrating gene-edited animal models with in situ structural biology, providing a new paradigm for dissecting the molecular architecture and physiological function of the axoneme. The combined approach highlights a promising frontier for future research into the structural basis of ciliary and flagellar function.
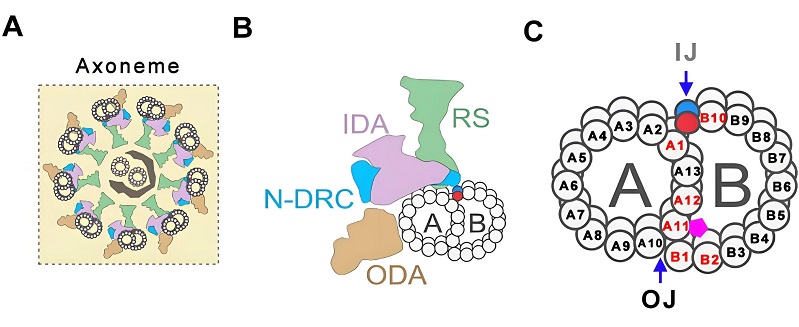
Figure 1. Schematic illustration of the axonemal structure of cilia/flagella and the A-B tubule connection within DMTs.
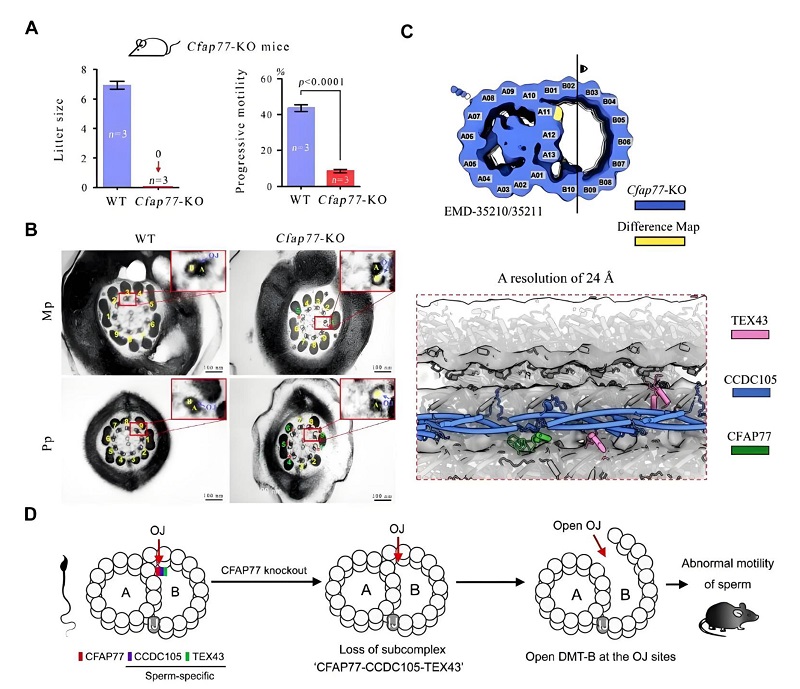
Figure 2. CFAP77 knockout leads to the loss of the CFAP77-CCDC105-TEX43 ternary complex and the opening of DMT B-tubules.
(Image by SUN Fei's group)
Article link:
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3003442
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: feisun@ibp.ac.cn
(Reported by Prof. SUN Fei's group)

