Newly Identified Protein Drives Colorectal Cancer Growth and Metastasis by Activating PRMT5 Complex
Protein arginine methyltransferase 5 (PRMT5), a key epigenetic regulator in colorectal cancer (CRC), requires assembly with the methylosome protein MEP50 into a hetero-octameric complex to exert its methyltransferase activity. However, the mechanisms governing the assembly of this complex have remained elusive.
A collaborative study led by the Institute of Biophysics of the Chinese Academy of Sciences and Zhengzhou University, published on October 15, 2025, in the Journal of Clinical Investigation, is the first to reveal that the previously uncharacterized protein C6orf223 promotes CRC growth and metastasis by facilitating the assembly of the PRMT5-MEP50 hetero-octameric complex with symmetric dimethyltransferase activity.
This discovery provides a new molecular target and therapeutic strategy for precision treatment of CRC.
The researchers found that the positively charged, arginine-rich N-terminal region of C6orf223 binds to a negatively charged surface groove located at the C-terminus of PRMT5. Moreover, C6orf223 forms dimers via a C178-mediated disulfide bond, which further promotes the assembly of the PRMT5-MEP50 hetero-octamer.
Both in vitro cell assays and in vivo orthotopic and metastatic CRC mouse models demonstrated that C6orf223 enhances tumor growth and metastasis, and that this function depends on its ability to modulate PRMT5 activity.
Through integrated analysis of ChIP-seq and RNA-seq data, the researchers identified the tumor suppressor gene GATA5 as a key downstream effector. They further confirmed three GATA5 target genes-WWTR1, FGFR1, and CLU-ultimately establishing the signaling axis: C6orf223 → PRMT5 activation → H4R3me2s enrichment → GATA5 downregulation → WWTR1/FGFR1/CLU upregulation.
Given the CRC-specific high expression of C6orf223 and the broad expression of PRMT5, the researchers developed a ferritin-nanocage-based siRNA delivery system (siC6orf223@tHFn(+)). Functional experiments showed that siC6orf223@tHFn(+) exhibits excellent biosafety and strong tumor-targeting specificity, and can significantly suppress CRC cell growth and metastasis.
This study not only identifies, for the first time, the protein properties of C6orf223 and elucidates the molecular mechanism by which it promotes PRMT5-MEP50 hetero-octamer assembly, but also uncovers a novel regulatory axis(C6orf223-PRMT5-H4R3me2s-GATA5) driving CRC progression. It further reveals a previously unknown tumor-suppressive role of GATA5 through inhibition of WWTR1, FGFR1, and CLU.
Most importantly, the development of the siC6orf223@tHFn(+) delivery system, which combines targeting specificity with safety, offers a promising new strategy for the precision treatment of CRC liver metastasis.
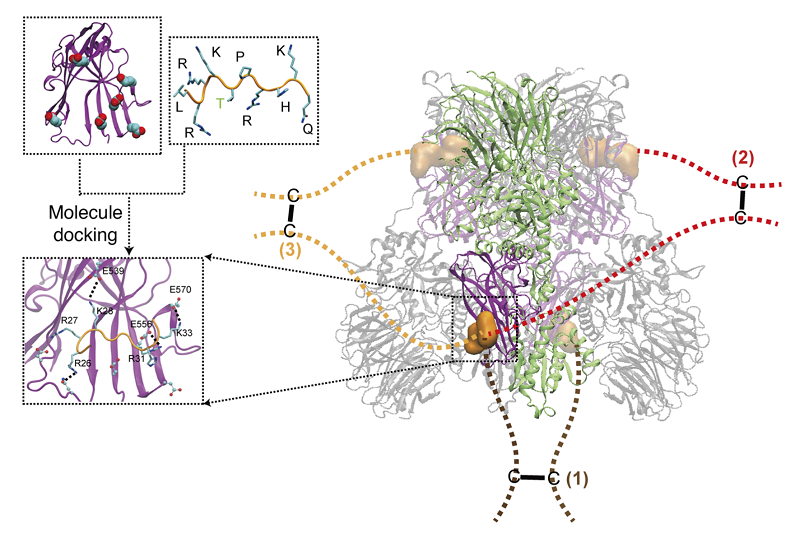
Figure: Schematic Illustration of C6orf223-Mediated Assembly of the PRMT5-MEP50 Hetero-Octamer
(Image by BU Pengcheng's group)
Article link: https://pmc.ncbi.nlm.nih.gov/articles/PMC12520688/
Contact: BU Pengcheng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: bupc@ibp.ac.cn
(Reported by Prof. BU Pengcheng's group)

