Scientists Unveil Key Structure of Type II Topoisomerase Capturing Transport-Segment DNA
On December 18, 2025, a research team led by Academician RAO Zihe, Associate Research Fellow CHEN Yutao, and Research Fellow LI Xuemei from the Institute of Biophysics of the Chinese Academy of Sciences published a research paper titled "Direct trapping of the transport-segment DNA by the central domain of type IIA topoisomerases" in Science Advances. This study presents the first cryo-electron microscopy (cryo-EM) structure of a type IIA DNA topoisomerase directly bound to transport-segment DNA (T-DNA). Using the T4 phage type II topoisomerase as a model system, the work reveals a long-missing intermediate structural state in the catalytic cycle of this enzyme class, providing critical insights for understanding its complete functional mechanism.
Type II DNA topoisomerases are essential enzymes that maintain chromosomal topological stability. They resolve DNA supercoils and entanglements arising during replication, transcription, and chromosome segregation by temporarily cleaving a "gate-segment DNA" (G-DNA) duplex, allowing another duplex (T-DNA) to pass through. Although structures of type II topoisomerases bound to G-DNA have been partially resolved in previous studies, the crucial step involving the capture and transport of T-DNA has long lacked direct structural evidence, representing a significant gap in the field.
This study reports the first three-dimensional structure, at a resolution of approximately 3 Å, of T-DNA stably captured within the central cavity of a type II topoisomerase under near-native solution conditions. The captured conformation is markedly different from the traditional G-DNA-bound state and more closely resembles the enzyme's apo conformation. Furthermore, electron density maps indicate signals from loosely associated G-DNA, suggesting this capture state likely occurs after G-DNA cleavage and re-ligation. Based on this structural evidence, the researchers propose that the enzyme may slide along the re-ligated yet transiently unreleased G-DNA to facilitate efficient successive catalytic cycles. Mutagenesis experiments further identified that the residue Arg375, located in the C-gate region, is crucial for T-DNA transport; altering its charge properties significantly inhibits the enzyme's supercoil relaxation activity. This finding suggests the C-gate region as a potential target for future inhibitors of type II topoisomerases.
In conclusion, these findings provide a key structural foundation for constructing a complete catalytic model of type II DNA topoisomerases and open new avenues for designing anti-infective and anti-tumor drugs targeting the T-DNA transport process.
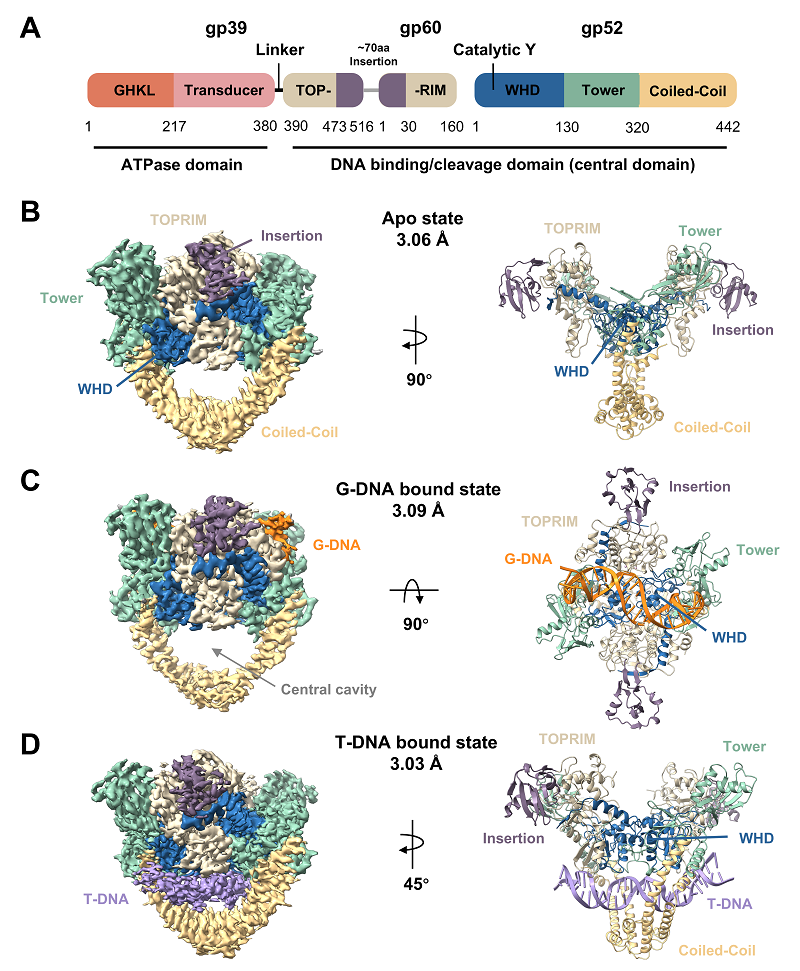
Figure: Cryo-EM structures of the central domain of T4 phage type II DNA topoisomerase in its Apo state, bound to gate-segment DNA, and bound to transport-segment DNA
(Image by RAO Zihe's group)
Article link: https://doi.org/10.1126/sciadv.adw2839
Contact: CHEN Yutao
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: chenyutao@ibp.ac.cn
(Reported by Prof. RAO Zihe's group)

