Cryo-EM Structures Reveal Conformational Dynamics Behind AP-4 Membrane Trafficking
Adaptor protein (AP) complexes, which couple cargo selection to vesicle formation, play central roles in intracellular vesicular trafficking. AP-4 is an important member of the AP family, and AP-4 dysfunction disrupts the transport of key cargo proteins such as ATG9A, leading to their abnormal retention within cells. However, how AP-4 is recruited to membranes, and how its structural features support this process, has remained largely unclear at the mechanistic level.
On January 21, 2026, a collaborative team led by Profs. FENG Wei and ZHAO Yan at the Institute of Biophysics of the Chinese Academy of Sciences, published a paper in Nature Communications. By combining cryo-electron microscopy, biochemical analyses, and cellular assays, the study systematically elucidates the conformational dynamics of the AP-4 core complex and uncovers the molecular mechanisms governing its membrane recruitment and cargo transport.
The researchers first reconstituted the soluble AP-4 core complex in vitro and determined its three-dimensional structure using single-particle cryo-EM. The structural data revealed that AP-4 is not a rigid, static assembly; instead, it exists in a dynamic equilibrium between "closed" and "open" conformational states.
Further analysis showed that this structural plasticity primarily stems from a relatively loose interface between the medium subunit μ4 and the core scaffold, providing a structural basis for conformation switching during function.
Subsequent cryo-EM reconstruction of the AP-4/ARF1 complex revealed that ARF1 does not activate AP-4 by locking it into a single conformation. Rather, ARF1 appears to modulate membrane recruitment within this dynamic conformational landscape.
Single-molecule FRET measurements independently validated the conformational flexibility of AP-4. Coupled with structure-guided mutagenesis of key interaction residues, the results confirmed that the AP-4-ARF1 interface is essential for efficient membrane recruitment.
The study further demonstrated that disrupting the intrinsic conformational equilibrium of AP-4 weakens the cooperative action of ARF1 and ATG9A during membrane recruitment, leading to aberrant intracellular localization of ATG9A.
Based on the integrated structural, biochemical, and cellular evidence, the researchers proposed a working model in which efficient AP-4 membrane recruitment and vesicle formation depend on the synergistic engagement of ARF1 and cargo proteins. Loss of AP-4's conformational dynamics disrupts this synergy and ultimately impedes vesicular transport.
By redefining how AP-4 is recruited to membranes from a conformational regulation perspective, this work provides important molecular insights into AP-4-related neurodevelopmental disorders. It also offers a structural and mechanistic framework for a deeper understanding of vesicular trafficking regulation.
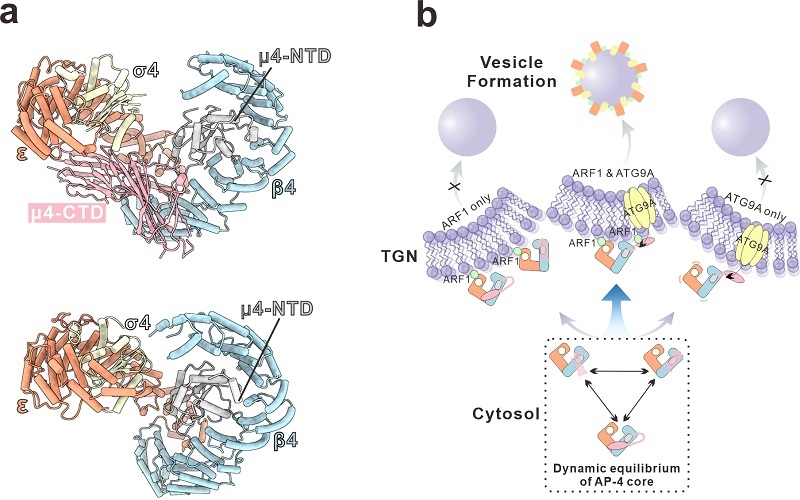
Figure: Cryo-EM structure of the AP-4 core complex and mechanistic model of its membrane recruitment
(Image by FENG Wei's group)
Article link: https://www.nature.com/articles/s41467-026-68679-8
Contact: FENG Wei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: wfeng@ibp.ac.cn
(Reported by Prof. FENG Wei's group)

