Scientists reveal the molecular mechanism for granzyme A targeting of GSDMB in lymphocyte pyroptotic killing
Pyroptosis is a form of lytic cell death mediated by the gasdermin (GSDM) family of pore-forming proteins, acting as a critical immune defense against infections or endogenous dangers. In innate immunity, the charter family member GSDMD features an autoinhibited two-domain architecture and is a substrate of both inflammasome-activated caspase-1 and lipopolysaccharide (LPS)-ligated caspase-4/5/11. Interdomain cleavage of GSDMD by the caspases disrupts the autoinhibition and unleashes the pore-forming activity in the GSDM-N domain to elicit pyroptosis. In cellular immunity, cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells use perforin to deliver serine protease granzymes (GZMs) into target cells to kill them. Perforin-GZM pathway was long-term considered to induce target cell apoptosis.
In 2020, Prof. SHAO Feng's group at the National Institute of Biological Sciences, Beijing discovered that another family member GSDMB is activated by granzyme A (GZMA) from cytotoxic lymphocytes. GZMA cleaves GSDMB at Lys244 within the interdomain linker, liberating the pore-forming activity to induce target cell pyroptosis. This finding suggests that lymphocyte killing is not always through apoptosis. GZMs are trypsin-like serine proteases, including GZMA, GZMB, GZMH, GZMK, and GZMM in humans. Upon perforin-mediated delivery into targets cells, GZMs act on various substrates to induce cell death or proinflammatory responses. Different GZMs have differentiations in their catalytic pocket, conferring the cleavage-site and substrate selectivity. GZMA is a Lys/Arg-specific protease with several in vitro substrates reported, but their cleavage-site sequences show little similarities. Thus, the substrate recognition mechanism for GZMA remains to be defined.
On Jan 26, 2026, a collaborative study led by Prof. DING Jingjin from the Institute of Biophysics of the Chinese Academy of Sciences and Prof. SHAO Feng from the National Institute of Biological Sciences, Beijing published a paper entitled "Exosite-mediated targeting of GSDMB by dimeric granzyme A in lymphocyte pyroptotic killing" in Immunity, revealing the molecular mechanisms for cytotoxic lymphocyte GZMA targeting of GSDMB.
The researchers discovered that human GZMA targets GSDMB via specific, high-affinity binding to its autoinhibitory GSDMB-C domain. This binding requires dimerization of GZMA, a unique property among human granzymes. They also determined the crystal structure of GZMA-GSDMB-C complex which shows a 2:2 stoichiometry, featuring a unique exosite at each of the two symmetric dimer interfaces in GZMA. The exosite engages a two-loop-organized site in the GSDMB-C domain, rendering a functional cleavage at Lys244 in GSDMB. They further found that mouse GZMA adopts a similar dimer structure, but its exosite is less efficient in engaging GSDMB. Mutation of the exosite enabled mouse GZMA to efficiently cleave and activate GSDMB.
This study solved the first GZM?substrate complex structure, and revealed that human GZMA uses a well-organized exosite, formed by two subunits via homodimerization, to specifically recognize a surface area in the GSDMB-C domain. This mode of action represents a substrate-recruiting mechanism for the granzyme family. Discovery of the GSDMB-recognition exosites well addresses a longstanding puzzle why GZMA appears as a homodimer among the granzyme family. This study also designed an exosite-restored mouse GZMA mutant that can cleave GSDMB and induce pyroptosis nearly as efficiently as human GZMA. The mutant can be genetically engineered into GSDMB transgenic mice to reconstitute a functional GZMA-GSDMB axis. This provides a highly valuable model for studying the pathophysiological functions of cytotoxic lymphocytes-mediated pyroptotic killing of target cells under various immunological contexts.
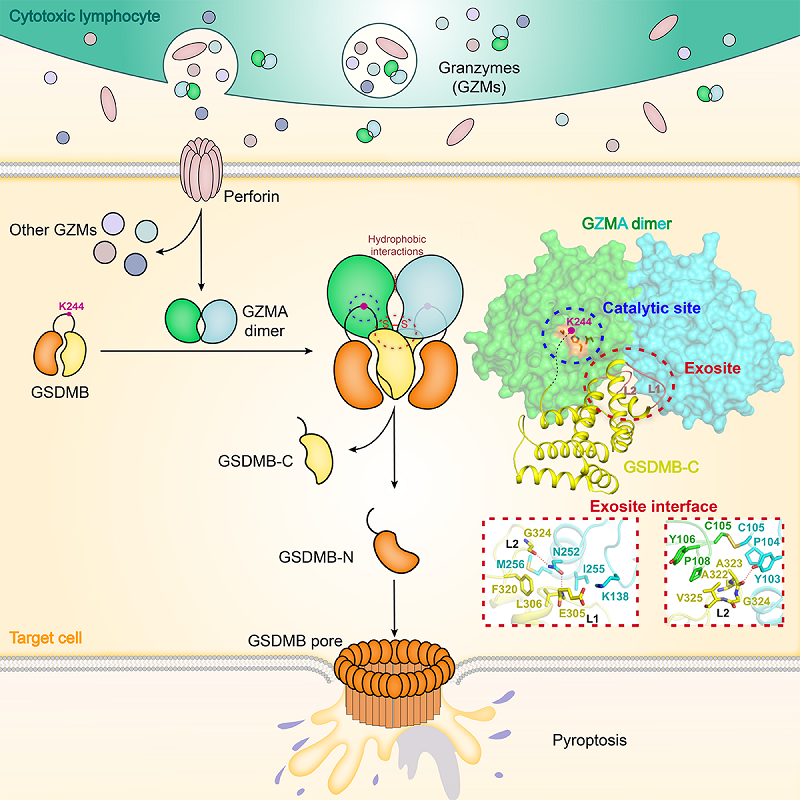
Figure:Schematic diagram of exosite-mediated targeting of GSDMB by dimeric granzyme A in lymphocyte pyroptotic killing
(Image by Ding Jingjin's group)
Article link: https://www.cell.com/immunity/fulltext/S1074-7613(25)00565-5
Contact: Ding Jingjin
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: jding@ibp.ac.cn
(Reported by Prof. Ding Jingjin's group)

