SIRT5 Protects Against MASLD Progression by Rewiring Hepatic Metabolism
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become one of the most prevalent chronic liver disorders worldwide, yet its molecular drivers of progression remain poorly understood.
A new study published on January 20, 2026 in Life Medicine reports that the metabolic enzyme SIRT5 as a key suppressor of MASLD progression through reshaping hepatic metabolic pathways. The work was led by researchers from the Institute of Biophysics of the Chinese Academy of Sciences, Beijing Tsinghua Changgung Hospital, and Chinese Academy of Medical Sciences & Peking Union Medical College.
Using dietary mouse models of MASLD, the researchers observed that Sirt5-deficient mice developed more severe steatosis and liver injury compared to controls. Histological analyses revealed increased lipid deposition and collagen staining, indicating heightened fibrotic remodeling.
To uncover the metabolic mechanisms underlying these phenotypes, the researchers performed multi-omics profiling, including transcriptomic, metabolomic, and proteomic analyses. These datasets collectively showed that SIRT5 regulates pathways linked to central carbon metabolism, amino acid catabolism, and redox homeostasis.
In Sirt5-deficient livers, dysregulation of these pathways led to elevated oxidative stress and inflammatory signaling, thereby promoting tissue damage and disease progression.
This study demonstrates the protective role of SIRT5 against metabolic dysregulation and chronic inflammation during MASLD progression. In MASLD mouse models, elevated free fatty acids and pro-inflammatory cytokines induced the expression of the E3 ubiquitin ligase TRIM21, which drove SIRT5 degradation.
Reduced SIRT5 levels led to increased lysine succinylation and malonylation on multiple metabolism-related and inflammation-associated proteins, impairing their functions. Restoring SIRT5 expression eliminated excessive protein acylation, improved glucose and lipid metabolism, and attenuated chronic inflammatory responses.
Together, the findings reveal that metabolic rewiring plays a critical role in liver disease development and identify SIRT5 as a protective factor against MASLD. By linking hepatic metabolism to inflammatory and fibrotic responses, the study provides a mechanistic basis for future therapeutic intervention strategies.
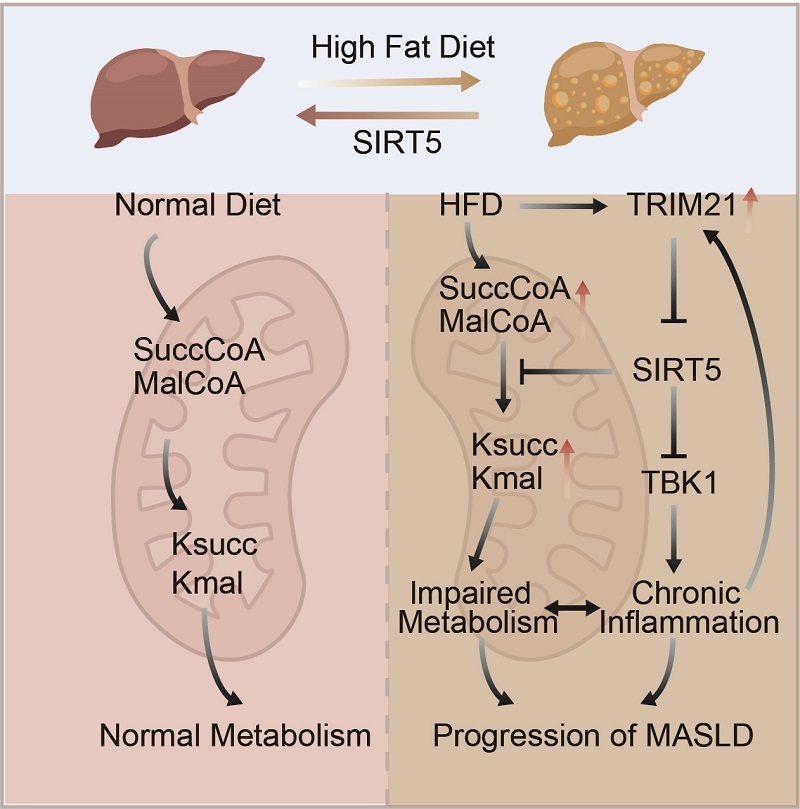
Figure: SIRT5 alleviates metabolic abnormalities and chronic inflammation in MASLD mice
(Image by WEI Taotao's group)
Article link:
https://academic.oup.com/lifemedi/advance-article/doi/10.1093/lifemedi/lnag001/8431721
Contact: WEI Taotao
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: weitt@ibp.ac.cn
(Reported by Prof. WEI Taotao's group)

