Cryo-EM Illuminates the Architecture and Disease Links of the Mammalian Sperm Axoneme
A research group led by Prof. SUN Fei from the Institute of Biophysics of the Chinese Academy of Sciences, published a review in Current Topics in Developmental Biology on January 23 that systematically summarizes key advances in the study of mammalian sperm axoneme architecture.
The review focuses on recent breakthroughs enabled by cryo-electron microscopy (cryo-EM) and in situ structural analyses, and highlights how these discoveries provide a new conceptual framework for understanding the mechanisms of sperm motility, elucidating the molecular basis of related diseases, and exploring potential intervention strategies.
The researchers emphasize that the sperm axoneme is not a static "structural scaffold," but rather a dynamic and highly sophisticated molecular machine built upon the canonical "9 + 2" microtubule framework, whose motile output depends on the precise coordination of multiple functional modules.
Dynein arms attached to the nine doublet microtubules act as ATP-driven molecular motors that directly power flagellar beating. Radial spokes function as signaling bridges that couple and transmit regulatory cues, while the nexin-dynein regulatory complex (N-DRC) serves dual roles in inter-doublet linkage and coordinated regulation. The central pair complex (CPC) operates as a critical control hub governing the pattern and directionality of axonemal motion.
A major advance highlighted in the review is the use of in situ cryo-EM to uncover structural asymmetry among the nine doublet microtubules of the axoneme.
In addition, in situ structural determination has enabled the integration of atomic-level structural information with genetic data, allowing researchers to define how gene mutations associated with asthenozoospermia specifically disrupt axonemal function. These insights provide a solid mechanistic explanation for certain forms of male infertility.
Looking ahead, the review concludes that a deeper understanding of axoneme assembly and its regulatory networks is expected to pave the way for precision diagnostic approaches and targeted therapeutic interventions for male infertility caused by specific protein defects.
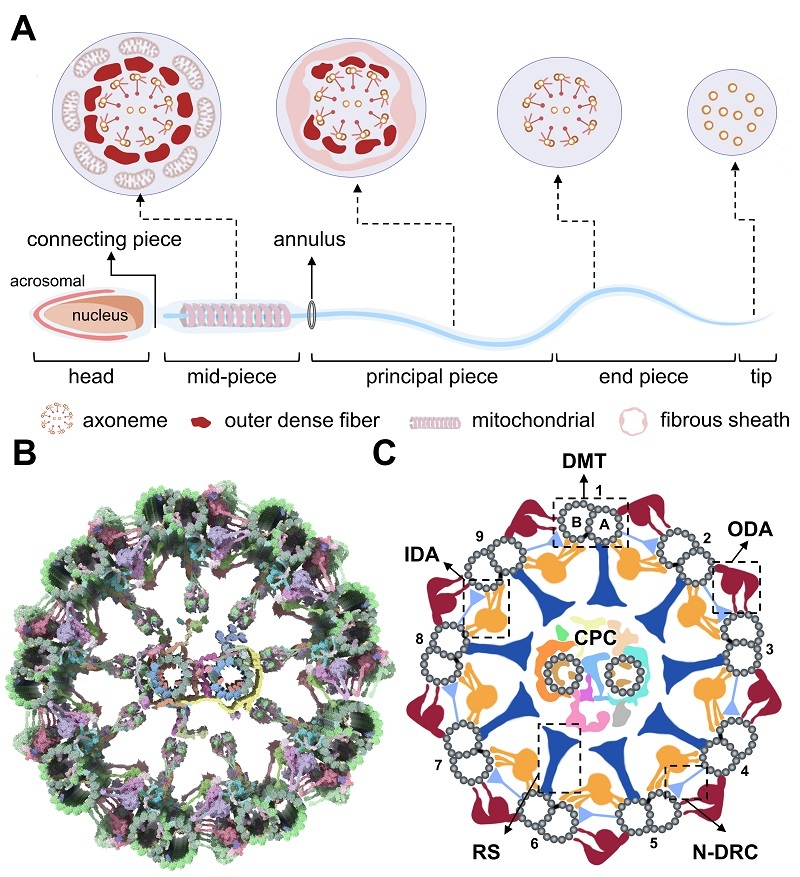
Figure. Overall morphology of a mammalian sperm and molecular and schematic models of the axonemal "9 + 2" microtubule scaffold
(Image by SUN Fei's group)
Article link: https://doi.org/10.1016/bs.ctdb.2026.01.002
Contact: SUN Fei
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
E-mail: feisun@ibp.ac.cn
(Reported by Prof. SUN Fei's group)

