
Yan Li, Ph.D, Prof.
-
Principal Investigator
State Key Laboratory of Cognitive Science and Mental Health, IBP
Research Interests: Molecular mechanisms of the advanced brain function with multiple approaches
Email: liyan@ibp.ac.cn
Tel: 010-64888533
Address: 15 Datun Road, Chaoyang District, Beijing, 100101, China
Chinese personal homepage
- Biography
1994 - 1998 BS in Physics and Biophysics, Peking University
1998 - 2003 Ph.D. in Biophysics, Chinese Academy of Sciences
2004 - 2007 Postdoctoral Fellow, UCSF
2007 - 2009 Research Assistant Professor, Northwestern University
2009 - Principal Investigator, Institute of Biophysics, Chinese Academy of Sciences
- Awards
- Membership in Academies & Societies
- Research Interests
We use Drosophila as animal model to explore the molecular mechanism of brain function, including neural developmental biology, molecular genetics, cognitive behavior of neural circuits etc. We will use the existing behavioral paradigm (eating, sleep and memory) as the basis to study effects of single genes on cell morphology, neural network, and the development of brain function.
1. The neural molecular mechanism of protein intake regulation
Feeding is one of the most conservative behaviors of living organisms. Although appetite signaling has been extensively studied, little is known about the mechanism of feeding ternmination. We firstly find the Drosophila protein specific satiety hormone - FIT, which is a kind of highly expressed gene in female fat body. As a secretory protein, FIT promotes insulin release in the Drosophila IPCs, thereby regulating the eating behavior. We screened 126 RNAi strains of GPCRs by behavioral experiments, and found that at least these three GPCRs were involved in protein intake. These studies reveal how nutrients can be perceived individually, and how the satiety signal is transmitted to the central nervous system.
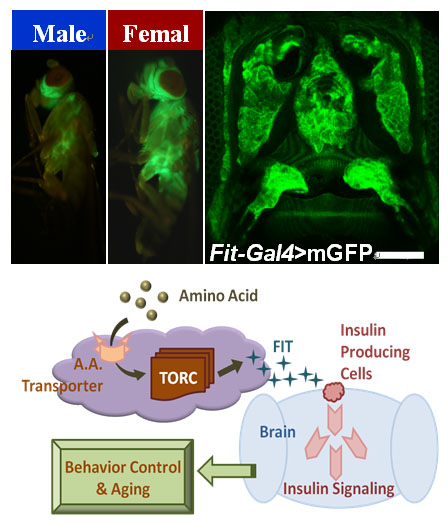
Fig. 1 FIT, a secreted protein, which is highly expressed in the fat body of female flies, and its signal transduction function in the control of protein intake.
2. Dendrite Development and Neurodegenerative Diseases
Dendrites are the major structure of neurons, which are responsible for receiving and integrating external info. We utilize the sensory neurons in Drosophila larvae to study the intracellular and extracellular factors in neurogenesis, polarization, and dendrite morphogenesis with genetic, molecular, and imaging approaches. We found that dendrites have directional growth like axons do, and epithelial derived Wingless signal plays essential role in dendrite patterning and efficient covering. We will continue to uncover the molecular pathway in dendrite routing, and to investigate its involvement in the pathogenesis of neurodegenerative diseases.
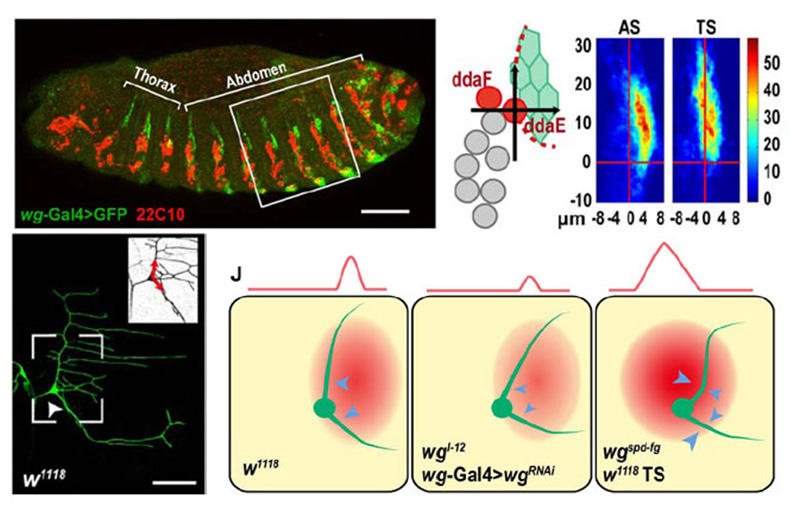
Fig.2 Epithelia Wingless is posterior-dorsal to the ddaE neuron, and plays a repulsive role in the directional growth of the primary dendrites of this neuron.
3. The mechanism of memory decline in Drosophila melanogaster
AMI (Aging-related Memory Impairment), which is caused by aging, is a ubiquitous phenomenon in insects and mammals. Memory erasure is an inhibitory modification, which is caused by interference. It is not clear whether memory erasure is affected in older animals. Previous studies have shown that anesthesia sensitive memory is significantly reduced in the elder. Using a memory retrieval paradigm in Drosophila, we found that the anti anesthesia memory was selectively erased, while the anesthetic sensitive memory was not affected. After 3 hours of erasure, the erased memory of young flies completely recovered, but not in the old fruit flies. The lack of a more severe erasure and memory recovery after deletion resulted in memory decline in older fruit flies.
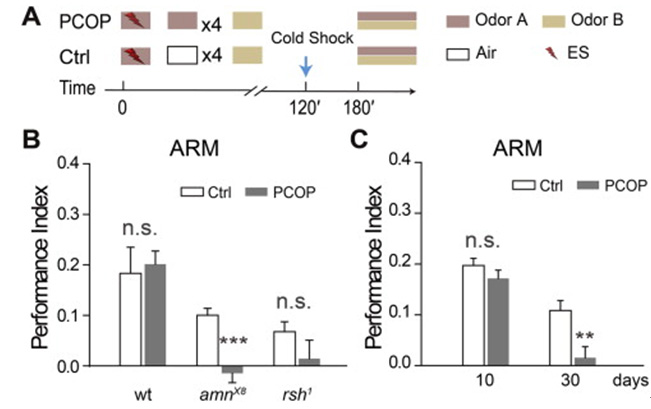
Fig. 3 Damaged ARM can be recovered in young fruit flies, but not in old fruit flies and amn mutants.
4. Neural Circus and Molecular Basis of Learning & Memory and Sleep
The Mushroom body (MB) is the brain center for olfactory learning & memory (L&M) as well as regulatory center of sleep in Drosophila. Our recent findings indicate that:
(1) There may have direct synaptic connection between the MB and the ellipsoid body (EB), one center for visual L&M, which could be the neural basis of cross-modal memory. We will further investigate the potential neural oscillation in memory consolidation.
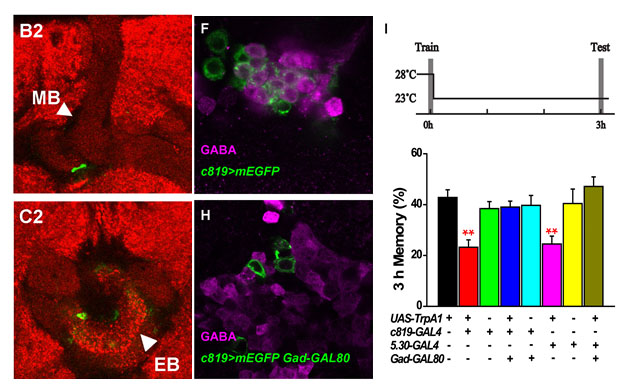
Fig.4. Direct synaptic connection between the MB and EB (left: green). GABAergic neurons (middle: violet) can be blocked by Gad-Gal80, in turn abolishes the inhibitory effect on memory consolidation (right).
(2) The core and surface regions of α/β MB lobes regulate sleep in opposite way, and both subgroups of neurons dependent on inhibitory Go signal and cholinergic output. Block Go signal can induce narcolepsy-like phenomenon in flies. It will be studied that whether these two adjacent subgroups are connected to each other, and whether this is the neural mechanism of sleep-wake switch.
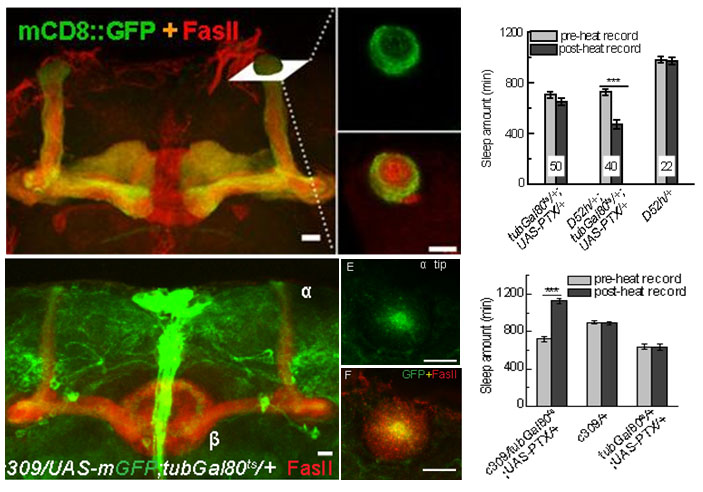
Fig.5. Opposite function of the core and surface regions of α/β MB lobes on sleep regulation.
(3) A pair of GABAergic APL neurons receives inhibitory regulation from dopaminergic neurons, and both of them project to the MB. This neural circuit ensures the efficient formation and regulation of a certain type of memory. Our next goal is to understand why this inhibitory regulation has such specificity, and whether it also crosstalks to another type of memory.
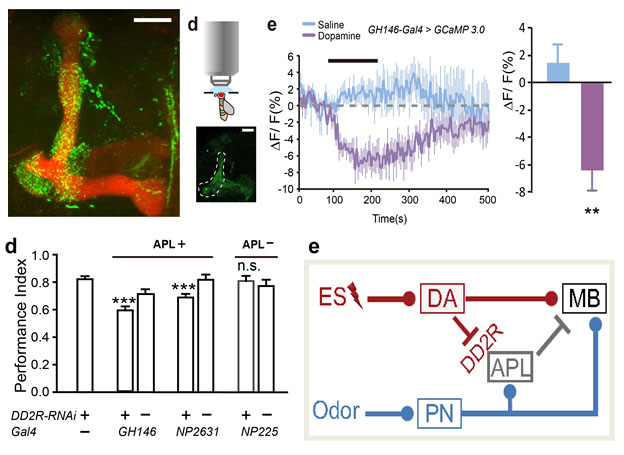
Fig.6. GABAergic APL neurons are inhibited by dopaminergic neurons through the direct synaptic connection (green) at the MB region (red). This DA-APL inhibition is essential for efficient memory formation.
5.The neural mechanism of nicotine addiction
Short-term exposure to nicotine induces positive effects in mice, monkeys and humans. However, the underlying neural basis and molecular mechanisms for these effects remain poorly understood. Here, using a video recording system, we find that acute nicotine administration induces locomotor hyperactivity in Drosophila. Our results reveal that in fruit flies, dopaminergic neurons mediate nicotine-induced acute locomotor hyperactivity in a sexually dimorphic manner, and Drosophila β1 nAChR subunit plays a crucial role in this nicotine response. These findings provide important insights into the molecular and neural basis of acute nicotine effects, and the underlying mechanisms may play conserved roles across species.
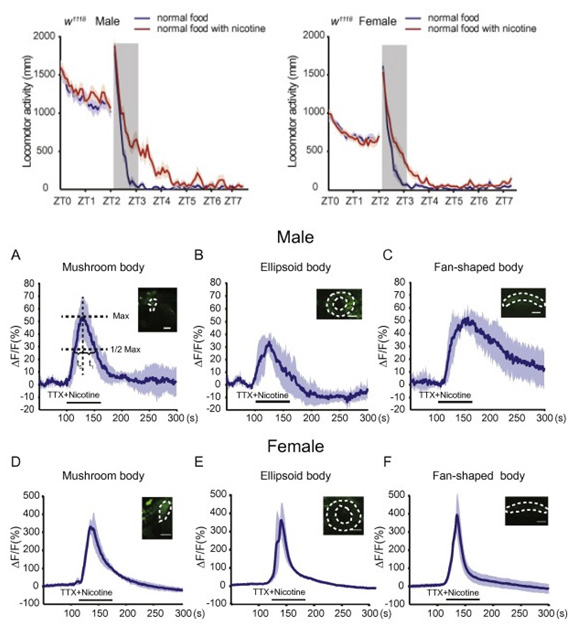
Fig.7. Dopaminergic neurons are widely activated upon nicotine perfusion in both sexes, while the response curve differs significantly between the sexes
- Grants
- Selected Publications
1. Xiaoyu Li*, Yang Yang*, Xiaobing Bai*, Xiaotong Wang, Houqi Tan, Yanbo Chen, Yan Zhu, Qili Liu, Mark N. Wu, Yan Li#. (2024) A brain-derived insulin signal encodes protein satiety for nutrient-specific feeding inhibition. Cell Rep. 43(6):114282.
2. Xiupeng Chen*, Jie Li*, Zhongbao Gao, Yang Yang, Wenqing Kuang, Yue Dong, Gek Huey Chua, Xiahe Huang, Binhua Jiang, He Tian, Yingchun Wang, Xun Huang, Yan Li, Sin Man Lam#, Guanghou Shui#.(2022) Endogenous ceramide phosphoethanolamine modulates circadian rhythm via neural-glial coupling in Drosophila. National science review. 9(12), nwac148.
3. Jianzhi Zeng*#, Xuelin Li*, Renzimo Zhang, Mingyue Lv, Yipan Wang, Ke Tan, Xiju Xia, Jinxia Wan, Miao Jing, Xiuning Zhang, Yu Li, Yang Yang, Liang Wang, Jun Chu, Yan Li, Yulong Li#. (2023) Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning. Neuron. 111(7), 1118-1135.e5.
4. Yanbo Chen*#, TongTong Liu*, Mengxia Niu, Xiaoting Li, Xinwei Wang, Tong Liu, Yan Li#. (2022) Epilepsy gene prickle ensures neuropil glial ensheathment through regulating cell adhesion molecules. iScience. 26(1), 105731.
5. Mengxia Niu, Xiaohang Zhang, Weihan Li, Jianxun Wang#, Yan Li#. (2021) dFRAME: A Video Recording-Based Analytical Method for Studying Feeding Rhythm in Drosophila. Front Genet. 12, 763200.
6. Mingmin Zhou*, Nannan Chen*, Jingsong Tian, Jianzhi Zeng, Yunpeng Zhang, Xiaofan Zhang, Jing Guo, Jinghan Sun, Yulong Li, Aike Guo#, Yan Li#. (2019) Suppression of GABAergic neurons through D2-like receptor secures efficient conditioning in Drosophila aversive olfactory learning. Proc. Natl. Acad. Sci. U.S.A. 116(11), 5118-5125.
7. Song Wu, Guangming Gan, Zhiping Zhang, Jie Sun, Qifu Wang, Zhongbao Gao, Meixiang Li, Shan Jin, Juan Huang, Ulrich Thomas, Yong-Hui Jiang, Yan Li, Rui Tian#, Yong Q Zhang#. (2017) A Presynaptic Function of Shank Protein in Drosophila. The Journal of neuroscience. 37(48), 11592-11604.
8. Jinghan Sun*, Chang Liu*, Xiaobing Bai*, Li Xiaoting, Zhiping Zhang, Yunpeng Zhang, Jing Guo, Yan Li#. (2017) Drosophila FIT is a protein-specific satiety hormone essential for feeding control. Nature Communication. 19;8:14161.
9. Qingqing Liu, Jingsong Tian, Xing Yang, Yan Li#, Aike Guo#. (2017) Locomotor Assay in Drosophila melanogaster. Bio-protocol. 7(10), e2283.
10. Yunpeng Zhang, Jing Guo, Aike Guo#, Yan Li#. (2016) Nicotine-induced acute hyperactivity is mediated by dopaminergic system in a sexually dimorphic manner. Neuroscience. 22;332:149-59.
11. Qingqing Liu, Xing Yang, Jingsong Tian, Zhongbao Gao, Meng Wang, Yan Li#, and Aike Guo#. (2016) Gap junction networks in mushroom bodies participate in visual learning and memory in Drosophila. Elife. 2016, 24;5.
12. Aike Guo#, Zhefeng Gong, Hao Li, Yan Li, Li Liu, Qingqing Liu, Huimin Lu, Yufeng Pan, Qingzhong Ren, ZhihuaWu, Ke Zhang, and Yan Zhu. (2016) Chapter "Vision, Memory and Cognition in Drosophila" in the book of "Learning and Memory: A Comprehensive Reference, 2nd edition".
13. Yan Li# and Zhiping Zhang. (2016) Emergence of intelligence. Review article. Prog Biochem Biophys. 43(4):348-353.
14. Xiaoting Li, Yan Wang, Huan Wang, Tongtong Liu, Jing Guo, Wei Yi, Yan Li#. (2016) Epithelia-derived wingless regulates dendrite directional growth of Drosophila ddaE neuron through the Fz-Fmi-Dsh-Rac1 pathway. Mol Brain. 29;9(1):46.
15. Yan Wang#, Huan Wang, Xiaoting Li, and Yan Li#. (2016) Epithelial microRNA-9a regulates dendrite growth through Fmi-Gq signaling in Drosophila sensory neurons. Dev Neurobiol. 76(2):225-37.
16. Chang Liu, Xiaobing Bai, Jinghan Sun, Xiaofan Zhang, and Yan Li#. (2015) Behavioral Switch of Food Preference upon Sugar Deficiency Is Regulated by GPCRs in Drosophila. J Genet Genomics. 20;42(7):409-12.
17. Nannan Chen, Aike Guo#, and Yan Li#. (2015) Aging accelerates memory extinction and impairs memory restoration in Drosophila. Biochem Biophys Res Commun. 460(4):944-8.
18. Wei Yi, Yunpeng Zhang, Yinjun Tian, Jing Guo, Yan Li#, and Aike Guo#. (2013) A Subset of Cholinergic Mushroom Body Neurons Requires Go Signaling to Regulate Sleep in Drosophila. Sleep. 36(12):1809-21.
19. Zhiping Zhang, Xiaoting Li, Jing Guo, Yan Li#, and Aike Guo#. (2013) Two Clusters of GABAergic Ellipsoid Body Neurons Modulate Olfactory Labile Memory in Drosophila. J Neurosci. 33(12):5175-81.
(From Yan Li, September 13, 2024)

