
Lei Wang, Ph.D, Prof.
-
Excellent Member of the Youth Innovation Promotion Association, CAS
Research Interests: ER redox homeostasis and human health
Email: wanglei@ibp.ac.cn
Tel: 010-64888501
Address: 15 Datun Road, Chaoyang District, Beijing, 100101, China
Chinese personal homepage
- Biography
2000.09 - 2004.06 B.Sc. in Biology (cum laude), College of Life Sciences, Wuhan University, Wuhan
2004.09 - 2009.06 Ph.D in Biochemistry and Molecular Biology, National Laboratory of Biomacromolecules, Institute of Biophysics (IBP), Chinese Academy of Sciences (CAS), Beijing (Supervisor: Prof. Chih-chen Wang)
2009.07 - 2011.12 Assistant Research Scientist, National Laboratory of Biomacromolecules, IBP, CAS
2011.02 - 2011.07 Visiting Scholar at San Raffaele Scientific Institute, Milan, Italy
2012.01 - 2019.12 Associate Research Professor, National Laboratory of Biomacromolecules, IBP, CAS
2020.01 - Professor, National Laboratory of Biomacromolecules, IBP, CAS
2020.01 - Professor, University of the Chinese Academy of Sciences
- Awards
08/2021-08/2025 Deputy Secretary General of Subcellular Structure and Function Subgroup, Biophysical Society of China
2021 Excellent Member of the Youth Innovation Promotion Association, CAS
2016 Member of the Youth Innovation Promotion Association, CAS
01/2015-12/2018 Committee Member of the Stress Physiology Society, China Association for Physiological Sciences
2009 "The Young Scientist Program Fellowship" of the 21st IUBMB and 12th FAOBMB International Congress of Biochemistry and Molecular Biology
2009 "Zhuli Yuehua" Award of CAS
2009 "Travel Award" of the 6th Asian Biophysics Association Symposium
2008 "Paper Award" from the Director of IBP
2004 Outstanding Graduates Award of Wuhan University
- Membership in Academies & Societies
- Research Interests
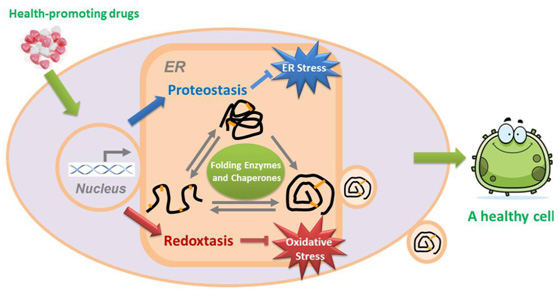
The endoplasmic reticulum (ER) is the largest part of the endomembrane system in eukaryotic cells, and is the factory for protein folding and processing. The ER contains a 'quality control' system, which is comprised of a large number of folding enzymes and chaperones, to maintain the protein homeostasis (proteostasis) in the ER. The ER provides an optimal redox environment for the correct folding of disulfide-containing proteins (e.g. secretory and membrane proteins), namely oxidative protein folding. The relationship between ER redox homeostasis (redoxtasis) and human health has attracted considerable attention in recent years. Dr. Lei Wang worked on the reconstitution of ER oxidative protein folding system during his Ph.D study, together with his mentor Prof. Chih-chen Wang. After graduation, he preferred to stay in the lab and extend his work to the regulation of ER redoxtasis and human health.
Recent Work Progresses:
1. Mechanism of oxidative protein folding in the ER
The ER sulfhydryl oxidase Ero1, protein-disulfide isomerase (PDI) and the ER peroxidase (e.g. GPx7) constitute the pivotal pathway for oxidative protein folding in the ER. The synergistic cooperation of the Ero1/PDI/GPx7 triad guarantees the efficiency and fidelity of oxidative protein folding. Our studies provide mechanistic insights into the ER oxidative protein folding system. Hopefully, future studies on oxidative protein folding will deepen our understanding of the principles of protein folding, and help design more efficient "cell factories" for the production of protein drugs.
2. Redoxtasis in the secretory pathway and human health
Emerging evidences have shown that the imbalance of ER redoxtasis is closely related to many diseases. Our studies have revealed that the Ero1/PDI/GPx7 oxidative protein folding system in the ER plays an important role in aging, cancer and cardiovascular diseases. Recently, it has been found that the oxidative protein folding system also exists on the cell surface, and it is involved in the regulation of many cellular processes (such as platelet activation, virus invasion and inflammatory response). The study of the redoxtasis in the secretory pathway will help to develop new intervention methods and provide new strategies for the prevention and treatment of related diseases.
3. Secretory pathway kinases and human diseases
The secretory pathway kinase represented by Fam20C is a new type of kinase family discovered in recent years. Our research found that Fam20C is a type II transmembrane protein in the Golgi apparatus. The secretion and kinase activity of Fam20C are governed by site-1 protease (S1P), a key regulator of cholesterol homeostasis. The maturation of Fam20C processed by S1P functions in osteoblast differentiation and mineralization. We also revealed a new mechanism for Fam20C to quickly and accurately regulate ER homeostasis by phosphorylating key enzymes under ER stress. In view of the broad substrate spectrum of the secretory pathway kinases, future studies on the regulatory mechanisms and physiological functions of these kinases will provide new ideas for our understanding of related diseases.
- Grants
1. NSFC Excellent Young Scientists Fund - "Regulation of endoplasmic reticulum stress" 2021/01-2023/12
2. NSFC General Program - "The role of endoplasmic reticulum homeostasis in the hyperhomocysteinemia-induced vascular endothelial cell dysfunction" 2018/01-2021/12
3. National Key R&D Program of China -- "Protein machinery for the dynamic interaction of cellular organelles" 2016-2021
4. NSFC General Program - "Endoplasmic reticulum oxidative stress and antioxidative response mediated by post-translational modifications of Ero1α" 2016/01-2019/12
5. NSFC General Program - "New insights into the mechanism of oxidative protein folding and its regulation in the endoplasmic reticulum" 2014/01-2017/12
6. NSFC Young Scientists Fund - "The working mechanism of electron transport system driven by sulfhydryl oxidase Ero1β in the endoplasmic reticulum of human cell" 2011/01-2013/12
- Selected Publications
1. Liu P, Wang X*, Sun Y, Zhao H, Cheng F, Wang J, Yang F, Hu J, Zhang H, Wang CC, Wang L* (2022) SARS-CoV-2 ORF8 reshapes the ER through forming mixed disulfides with ER oxidoreductases. Redox Biol 54:102388.
2. Wang L*, Wang X, Lv X, Jin Q, Shang H, Wang CC, Wang L* (2022) The extracellular Ero1α/PDI oxidative folding system regulates platelet function by increasing glutathione reduction potential. Redox Biol 50: 102244
3. Qiao X, Zhang Y, Ye A, Zhang Y, Xie T, Lv Z, Shi C, Wu D, Chu B, Wu X, Zhang W, Wang P, Liu GH, Wang CC, Wang L*, Chen C* (2022) Reductive stress in the endoplasmic reticulum caused by Ero1α S-nitrosation accelerates senescence. Free Radic Biol Med 180: 165-178.
4. Fan F, Zhang Q, Zhang Y, Huang G, Liang X, Wang CC, Wang L*, Lu D* (2022) Two protein disulfide isomerase subgroups work synergistically in catalyzing oxidative protein folding. Plant Physiol 188: 241-254.
5. Chen X, Zhang J, Liu P, Wei Y, Wang XE, Xiao J, Wang CC, Wang L* (2021) Proteolytic regulation of secretory pathway kinase Fam20C by site-1 protease. Proc Natl Acad Sci U S A 118: e2100133118.
6. Yan Y, Wu X, Wang P, Zhang S, Sun L, Zhao Y, Zeng GY, Liu B, Xu G, Liu H, Wang L*, Wang X* and Jiang C* (2020) Homocysteine promotes hepatic steatosis by activating the adipocyte lipolysis in a HIF1α-ERO1α-dependent oxidative stress manner. Redox Biol 37: 101742.
7. Yu J, Li T, Liu Y, Wang X, Zhang J, Wang Xe, Shi G, Lou J, Wang L, Wang CC, Wang L* (2020) Phosphorylation switches protein disulfide isomerase activity to maintain proteostasis and attenuate ER stress. EMBO J 39: e103841
(Highlighted by EMBO J 'News & Views'; Recommended by F1000Prime)
8. Fan F, Zhang Y, Huang G, Zhang Q, Wang CC, Wang L*, Lu D* (2019) AtERO1 and AtERO2 exhibit differences in catalyzing oxidative protein folding in the ER. Plant Physiol 180:2022-2033.
9. Zhang Y, Li T, Zhang L, Shangguan F, Shi G, Wu X, Cui Y, Wang Xe, Wang X, Liu Y, Lu B, Wei T, Wang CC, Wang L* (2019) Targeting the functional interplay between endoplasmic reticulum oxidoreductin-1α and protein disulfide isomerase suppresses the progression of cervical cancer. EBioMedicine 41:408-419.
10. Wu X, Zhang L, Miao Y, Yang J, Wang X, Wang CC, Feng J*, Wang L* (2019) Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol 20: 46-59.
11. Zhang J, Zhu Q, Wang Xe, Yu J, Chen X, Wang J, Wang X, Xiao J, Wang CC, Wang L* (2018) Secretory kinase Fam20C tunes endoplasmic reticulum redox state via phosphorylation of Ero1α. EMBO J 37: e98699.
12. Fang J, Yang J, Wu X, Zhang G, Li T, Wang Xe, Zhang H, Wang CC, Liu GH*, Wang L* (2018) Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 17: e12765.
13. Li H, Yang K, Wang W, Niu Y, Li J, Dong Y, Liu Y, Wang CC, Wang L*, Liang H* (2018) Crystal and solution structures of human protein disulfide isomerase-like protein of the testis (PDILT) provide insight into its chaperone activity. J BiolChem 293, 1192-1202.
14. Fan F, Zhang Y, Wang S, Han Y, Wang L*, Lu D* (2018) Characterization of the oxidative protein folding activity of a unique plant oxidoreductase, Arabidopsis protein disulfide isomerase-11. Biochem Biophys Res Commun 495:1041-1047.
15. Niu Y, Zhang L, Yu J, Wang CC* and Wang L* (2016) Novel roles of the non-catalytic elements of yeast protein-disulfide isomerase in its interplay with endoplasmic reticulum oxidoreductin 1. J Biol Chem 291:8283-8294.
16. Zhang L, Niu Y, Zhu L, Fang J, Wang Xe, Wang L* and Wang CC* (2014) Different interaction modes for protein-disulfide isomerase (PDI) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1α (Ero1α). J Biol Chem 289:31188-31199.
17. Wang L*, Zhang L, Niu Y, Sitia R and Wang CC* (2014) Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1α to promote oxidative protein folding. Antioxid Redox Signal 20: 545-556.
18. Wang L, Zhu L and Wang CC (2011) The Endoplasmic Reticulum Sulphydryl Oxidase Ero1β Drives Efficient Oxidative Protein Folding with Loose Regulation. Biochem J 434:113-121.
19. Wang L, Li SJ, Sidhu A, Zhu L, Liang Y, Freedman RB, and Wang CC* (2009) Reconstitution of human Ero1-Lα/protein disulfide isomerase oxidative folding pathway in vitro: position-dependent differences in role between the a and a' domains of protein disulfide isomerase. J Biol Chem 284: 199-206.
Invited Reviews (*Corresponding author):
1. Wang L* and Wang CC (2022) Oxidative protein folding fidelity and redoxtasis in the endoplasmic reticulum. Trends Biochem Sci doi.org/10.1016/j.tibs.2022.06.011.
2. Wang L*, Yu J, Wang CC (2021) Protein disulfide isomerase is regulated in multiple ways: Consequences for conformation, activities and pathophysiological functions. BioEssays 43: e2000147.
3. Wang L, Wang X and Wang CC* (2015) Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free Radical Bio Med 83:305-313.
Full publication list: https://scholar.google.com/citations?hl=en&user=NUni5KMAAAAJ
(From Lei Wang, July 21, 2022)

