Chinese scientists reveal the molecular mechanism of blocking host death receptor signaling by bacterial pathogen
On April 10, 2019, a research article entitled "Structural and functional insights into host death domains inactivation by the bacterial arginine GlcNAcyltransferase effector" was published in Molecular Cell by a collaborative study of Wang Dacheng - Ding Jingjin's group from the Institute of Biophysics, Chinese Academy of Sciences, Shao Feng's group from National Institute of Biological Sciences, Beijing, and Li Shan's group from Huazhong Agricultural University. They deciphered the precise molecular mechanism of blocking host death receptor signaling by Enteropathogenic E. coli (EPEC) arginine GlcNAcyltransferase NleB.
Employing specialized effectors to manipulate or block host signaling pathways is a common strategy used by bacterial pathogen to facilitate infection and evade host immune defenses. Death receptor signaling is an emerging strategy for host counteracting bacterial infection through stimulation of NF-κB-mediated transcriptional response for inflammation and activation of caspase-mediated programmed apoptosis. Enteropathogenic E. coli (EPEC) is a harmful bacterial pathogen which causes diarrhea, enteritis, and other intestinal diseases. In 2013, Dr. Shao Feng's group identified a type III secretion system effector of EPEC, NleB which catalyzes the attachment of N-acetylglucosamine (GlcNAc) onto a crucial arginine of the death domain (DD) and there by disrupts death-domain interactions. The modification blocks NF-κB activation, apoptosis, and necroptosis in EPEC-infected cells and facilitates bacterial survival and colonization in mouse colon. NleB-catalyzed arginine GlcNAcylation represents an unprecedented post-translation modification (PTM) in host cells. However, the precise mechanism underlying NleB recognition of death-domain substrates and catalysis of arginine GlcNAcylation is unknown.
In this study, the researchers determined crystal structures of NleB alone, NleB in complex with death-domain substrate (FADD-DD) and the sugar donor, as well as arginine-GlcNAcylated death domains (TRADD-DD and RIPK1-DD). These provide structural snapshots for all major steps in NleB-catalyzed arginine GlcNAcylation of death domains. The structural data, together with in vitro biochemical analyses and in vivo mouse infection assays, reveal the detailed molecular mechanism for NleB functioning.
Extensive hydrogen-bond and electrostatic interactions at the intermolecular interface confer NleB with specific recognition and high affinity to a subgroup of death-domainsubstrates. The side chain of acceptor arginine stretches into a narrow cleft in NleB, generating a bidentate hydrogen-bond interaction between the arginine guanidinium and the carboxyl group of Glu253 of NleB. Glu253 acts as the base to deprotonate the guanidinium in the arginine substrate. Then the arginine nucleophilically attacks the C1 atom of UDP-GlcNAc, forming an oxocarbenium ion-like transition state that progresses to SN2-like displacement of the leaving group UDP. In other words, NleB employs an inverting sugar-transfer mechanism to convert α-anomeric configuration of C1 in UDP-GlcNAc intoa β-glycosidic linkage of GlcNAcylated arginine. Moreover, NleB is a broad-spectrum inhibitor of death receptor signaling due to its pleotropic blocking of death-domain interactions.
This study expands our knowledge about bacterial effectors manipulation of host signaling pathways via novel PTM, and guides NleB-based drug development for antagonizing EPEC infection. More importantly, as a novel type of PTM, NleB-catalyzed arginine GlcNAcylation is quite different from other known glycosylation in host cells. The findings of this study provide a comprehensive and in-depth understanding of the enzymatic reaction mechanism of arginine GlcNAcylation. Modification and application of NleB will greatly facilitate us to study the complicated functional and regulatory mechanisms of host death receptor signaling.
The research work was supported by the National Key Research and Development Programs of China, the Strategic Priority Research Program of CAS, and the program Youth Innovation Promotion Association of CAS.
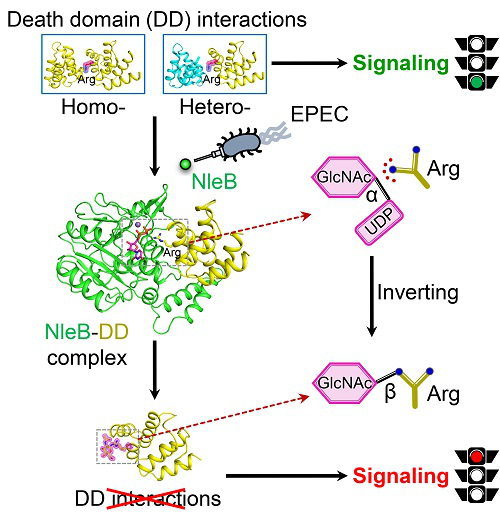
Sketch map of precise molecular mechanism of blocking host death receptor signaling by Enteropathogenic E. coli (EPEC) arginine GlcNAcyltransferase NleB.
Article Link: https://www.cell.com/molecular-cell/fulltext/S1097-2765(19)30232-1

