New insights into membrane trafficking regulated by ER fusion protein
The study entitled "Atlastin-mediated membrane tethering is critical for cargo mobility and exit from the endoplasmic reticulum" was published online on June 25, 2019. In this study, the endoplasmic reticulum (ER) fusogen atlastin (ATL) was found to be involved in regulating cargo mobility and COPII formation in the ER. These findings provide important insight into the physiological role of the tubular ER network.
In eukaryotic cells, the ER forms a complex network of continuous sheets and tubules. Tubules are shaped by a class of integral membrane proteins, the reticulons and REEPs, and subsequently connected in the form of 3-way junctions by dynamin-like GTPase atlastin (ATL). Deletion or mutation of ATL in mammalian cells resultsinlong unbranched ER tubules indicative of a lack of fusion between tubules, and mutation of human ATL1 is linked to hereditary spastic paraplegia. Hu's previous work demonstrated that ATL and its homologs mediate fusion of the ER, particularly the tubular network, but the specific physiological functions of the tubular ER networks remain unclear.
After folding and proper modification, secretory proteins and transmembrane proteins of other endosystem organelles will be delivered to Golgi by COPII coated vesicles. In ATL-deleted cells, COPII formation is drastically reduced in cell periphery, and ER export of cargo proteins becomes defective. While ER exit site initiation is not affected, many of the sites fail to recruit COPII subunits. Subsequently, in vitro vesicular release assay revealed that the efficiency of cargo packaging into COPII vesicles is significantly reduced in cells lacking ATLs, or when the ER is transiently fragmented. In addition, cargo is less mobile in the ER in the absence of ATL. Interestingly, the cargo mobility and COPII formation can be restored by ATL R77A, which is capable of tethering, but not fusing, ER tubules. These findings suggest that ATL-mediated membrane tethering plays a critical role in maintaining the necessary mobility of ER contents to allow efficient packaging of cargo proteins into COPII vesicles. It has been shown that membrane tension affects mobility of membrane components, including lipids and embedded proteins. The activity of ATL, particular membrane tethering, may contribute to maintain lateral membrane tension.
Prof. Junjie Hu at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences (CAS) and Prof. Yusong Guo at Hong Kong University of Science and Technology are co-corresponding authors. Liling Niu from Hu's team and Tianji Ma from Guo's team share the first authorship. This work was supported by the National Natural Science Foundation of China, the National Key Research and Development Program, and the Strategic Priority Research Program (pilot study) "Biological basis of aging and therapeutic strategies" of the Chinese Academy of Sciences.
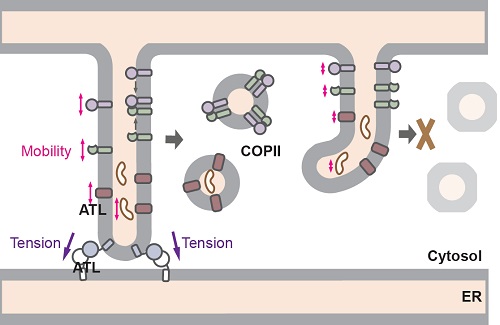
A model for the role of ATL in COPII formation
(Imaged by Dr. Hu Junjie's group)
Article link: https://www.pnas.org/content/early/2019/06/24/1908409116
Contact: Junjie Hu
Institute of biophysics, Chinese Academy of Sciences
Beijing100101, China
Tel: (86)-10-64886852
Email: huj@ibp.ac.cn
(Reported by Dr.Hu Junjie's group)

