WANG Yanli's group elucidates the mechanisms of DNA cleavage by Cas9 and its inhibitionstate by AcrIIC3
CRISPR-Cas system is an adaptive immune system of bacteria and archaea to prevent against the foreign nucleic acids. Cas9, the signature Cas protein in type II CRISPR-Cas system, is an RNA-guided DNA endonuclease. Cas9 cleaves target dsDNA via its RuvC and HNH domains, and it is widely used for genome engineering applications. In currently available DNA-bound, pre-cleavage Cas9 structures, the active site of the HNH domain is distant from the cleavage site of the target strand. The HNH domain is in the inactive state in all previously reported structures. To completely understand the mechanism of targetstrand cleavage by the HNH domain, it is critical to elucidate the structures of DNA-bound Cas9 complexes in their catalytically competent states. Moreover, the most commonly used SpyCas9 has1, 368 amino acids, limiting its delivery along with sgRNA within a single AAV vector.
Importantly, two recently characterized Cas9 homologs from Neisseria meningitides have been developed as a compact and high-accuracy genome-editing tool. In 2016, three anti-CRISPR proteins(AcrIIC1-3) were reported to inhibit NmeCas9 by Karen Maxwell’s group from University of Toronto. AcrIIC2 was thenfound to prevent the assembleof the Cas9-sgRNA complex by a collaborative work by WANG Yanli’s group from Institute of Biophysicsand Karen Maxwell’s group. However, the complete molecular mechanisms for the DNA cleavage by NmeCas9s and the inhibition of the DNA cleavage by AcrIIC3 are still unknown.
On October 24, 2019, a research article entitled “Structures of Neisseria meningitidis Cas9 Complexes in Catalytically Poised and Anti-CRISPR Inhibited States” was published in Molecular Cell by WANG Yanli’s group and Erik Sontheimer’s group from University of Massachusetts Medical School. They reported structures of the NmeCas9-sgRNA binary complex, a seed-matched (Fig. 1A), a fully paired pre-catalytic, a fully paired catalytically poised (Fig. 1B), and a post-TS-cleavage DNA-bound NmeCas9 ternary complexes. These structures represent 5 distinct states of NmeCas9 along its DNA cleavage pathway, showing the processes of DNA cleavage step by step as a movie. These results provide a high-resolution view of the engagement of a Cas9 HNH domain with the guide-target heteroduplex, and show important structural rearrangement of the REC2 and HNH domains of NmeCas9 during the target DNA binding and cleavage.The HNH domain is flexible and exhibits a conformational equilibrium that usually favors positioning distant from the cleavage site. Authors also found that HNH mutants, which have higher binding affinity with the target, have enhanced DNA cleavage activity.
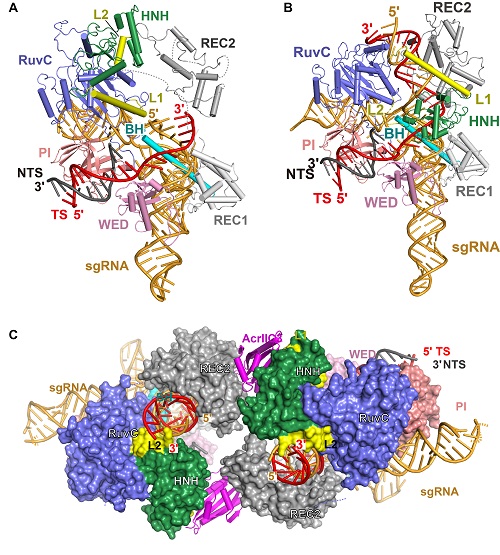
Figure 1. Structures of seed-matched (A), a fully paired catalytically poised (B), DNA-bound NmeCas9 complexes, and the NmeCas9-sgRNA-DNA-AcrIIC3 complex (C).
(Imaged by Dr. WANG Yanli's group)
WANG Yanli's group also reported two structures of AcrIIC3-bound NmeCas9-sgRNA complexes with or without dsDNA (Fig. 1C). These structural studies reveal that two AcrIIC3 proteins tether two NmeCas9 proteins together, and the AcrIIc3 traps the HNH domain away from the cleavage site, thus preventing DNA cleavage by Cas9 (Fig. 2). Together, these structures provide insights into Cas9 domain rearrangements, guide-target engagement, cleavage mechanism, and anti-CRISPR inhibition, facilitating the optimization of these genome-editing platforms.
This work was supported by grants from the Chinese Ministry of Science and Technology, the Natural Science Foundation of China and the Chinese Academy of Sciences. The staffs of the BL-17U1 and BL-19U1 beamlines at the Shanghai Synchrotron Radiation Facility and the BL41XU beamline at SPring-8 have provided technical supports to this work.
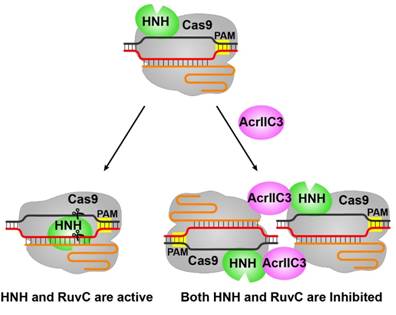
Figure 2. The model of the DNA cleavage by Cas9 and the inhibition of Cas9 activity by AcrIIC3.
(Imaged by Dr. WANG Yanli's group)
Article link: https://www.cell.com/molecular-cell/fulltext/S1097-2765(19)30730-0
Contact: Yanli Wang
Institute of biophysics, Chinese Academy of Sciences
Beijing 100101, China
Tel: (86)-10-64881316
Email: ylwang@ibp.ac.cn
(Reported by Dr. WANG Yanli's group)

