A discovery reveals communications between lysosome and ECM
Lysosomes degrade extra- and intra-cellular macromolecules delivered by endocytosis, phagocytosis or autophagy and recycle the resulting catabolites for reutilization in cellular metabolism. Lysosomes also serve as signaling hubs to integrate nutritional, energy and growth factor information and relay it to key regulatory modules, such as mTOR, which is docked on the lysosomal surface. Thus, lysosomes play crucial roles in cell homeostasis by acting as centers of digestion, recycling and signaling. However, very little is known about how lysosomes contribute to tissue homeostasis during development.
On Nov 14, Developmental Cell journal published a research from Dr. WANG Xiaochen’s Lab from National Laboratory of Biomacromolecules located in the Institute of Biophysics, CAS. Here, her lab uses nematode C. elegans as a model system to investigate lysosome function and regulation during C. elegans development. In this research, they described and analyzed an interesting lysosomal morphological change specifically in worm epidermis during development; and their study reveals a communication between lysosomes in cell and extracellular matrix structure-cuticle.
C. elegans develops through four consecutive larval stages (L1-L4) separated by molts. During each molt, worms synthesize and secrete a new exoskeleton called cuticle underneath the existing one, followed by their separation (apolysis) and the shed of the old exoskeleton (ecdysis). Thus, the apical ECM is remodeled during molting. They found that worms at each molting stage contain extensive tubular lysosomes in the epidermis, whereas vesicular lysosomes are predominant when animals exit molt. Also, lysosome number and lysosome dynamics is increased, and lysosome acidification/maturation is accelerated during molting. Consistent with this, they observed greatly increased lysosomal degradation activity in molting animals.
Steps further, scientists investigated the mechanism behind this interesting process. Apolysis is achieved by disruption of the attachments (FOs) between epidermis and cuticle, which must be remodeled during molting. They found that during molting, the apical ceHD MUP-4 is downregulated, and the relative position of cuticle and epidermis is altered. Furthermore, when epidermal structure is damaged upon inactivation of FO components during intermolt stage, they could see similar lysosomal properties as those in molting worms. This suggests that the epidermal structural change during molting is the upstream signal that induce lysosome activation.
At last, it has been shown the lysosome V-ATPases transcription are upregulated upon epidermal structural change when molting or when FOs disrupted. V-ATPases is the proton pump that acidifies lysosome. Thus, upregulation of V-ATPases expression mediates lysosome activation during molting. Transcription factors STAT/STA-2 and GATA/ELT-3 are both required for the upregulation of V-ATPases in this process.
This research began with an interesting observation during routine work. Searching for mechanism underlying reveals a cross-tissue regulation of lysosome activities.
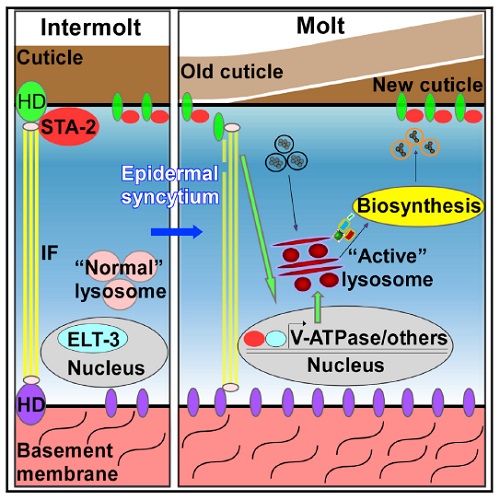
Figure 1. ECM remodeling and regulation of lysosome during C. elegans molting.
(Imaged by Dr. WANG Xiaochen's group)
Contact: XiaochenWang
Institute of biophysics, Chinese Academy of Sciences
Beijing100101, China
Tel: (86)-10-64888878
Email: wangxiaochen@ibp.ac.cn
(Reported by Dr. WANG Xiaochen's group)

