Researchers Elucidate Mechanism of REV7 Recruitment by SHLD3 during DNA Double-Strand Break Repair
DNA double-strand breaks (DSBs) are extremely vicious DNA lesions that may cause carcinogenesis or cell death if not properly repaired. In vertebrate cells, two main repair pathways: non-homologous end-joining (NHEJ) and homologous recombination (HR) are employed in eliminating the cytotoxic DSBs and thereby ensuring genomic integrity. The decision-making process of repair pathways is a critical step during DSB response, which is spearheaded by 53BP1 and its downstream effectors.
A recently characterized four-subunit complex, named Shieldin, acts downstream 53BP1 to protect DNA end from resection and facilitate NHEJ repair. SHLD3 is the most apical subunit within complex and it constitutes the DSB recruitment module of Shieldin along with REV7. SHLD3 and REV7 are essential for correct localization of Shieldin at DSB sites. But how they interact with one another remains unknown. A new study by Chinese and American researchers has now revealed the underlying mechanism. This research enhances understanding of how the DSB recruitment module assembles within Shieldin complex.
The study was conducted by Dr. ZHOU Zheng's group from the Institute of Biophysics (IBP) of the Chinese Academy of Sciences and Dr. GONG Zihua's group from Cleveland Clinic Lerner Research Institute in America. Results were published online in Journal of Biological Chemistry on Dec. 3, 2019 in an article entitled "Structural basis for shieldin complex subunit 3–mediated recruitment of the checkpoint protein REV7 during DNA double-strand break repair".
ZHOU's team firstly identified the minimal REV7-binding domain (RBD) in SHLD3 after several rounds of screening. Through Isothermal Titration Calorimetry (ITC), SHLD3-RBD was shown to have a high-affinity binding ability to REV7 (with low-nanomolar affinity). Then the researchers successfully assembled stable SHLD3-REV7 complex and determined high-resolution complex structures by using X-ray crystallography.
The structures revealed that SHLD3-RBD binds REV7 in a unique ladle-shaped conformation, with its N-terminal loop and C-terminal α-helix (αC-helix) acting as "stem" and "base", respectively. Through extensive in vitro and in vivo binding analyses, the scientists found that both parts of SHLD3-RBD are indispensable for REV7 recognition.
In addition, via surface plasmon resonance (SPR) assay, the researchers presented a binding kinetic view of REV7-SHLD3 interaction. The results showed that the "safety-belt" region, which plays a role in binding other proteins, is essential for SHLD3-REV7 binding, as this region retards the dissociation of the RBD from the bound REV7.
This study revealed the molecular basis of the SHLD3-REV7 interaction, provided critical insights into how SHLD3 recruits REV7, and paved the way for medicine development towards cancer treatment.
The project was funded by the Chinese Ministry of Science and Technology, the National Natural Science Foundation of China and the Strategic Priority Research Program. The staff of the BL-17U1 and BL-19U1 beamlines at the Shanghai Synchrotron Radiation Facility and the Institute of Biophysics have provided technical supports to this work.
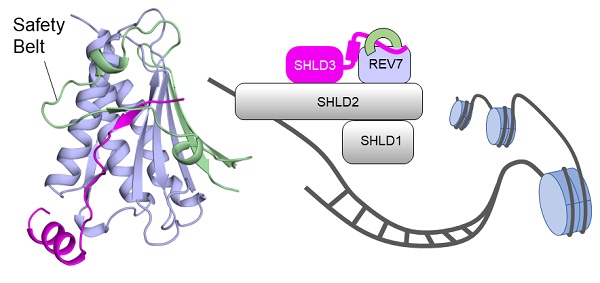
Figure: Overall structure of SHLD3-REV7 complex and their roles in DSB repair
(The image by Dr. ZHOU Zheng's lab)
The web link for this paper is http://www.jbc.org/content/early/2019/12/03/jbc.RA119.011464.abstract
Contact: ZHOU Zheng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889862
Email: zhouzh@ibp.ac.cn
(Reported by Dr. ZHOU Zheng's group)

