Researchers Reveal Mechanism of Swc5 Facillitates the Chromatin Deposition of Histone Variant H2A.Z
Eukaryotic DNA is packaged into chromatin by wrapping over histone octamer (including two copies of histone H2A, H2B, H3, and H4). Eukaryotic cells mobilize histones at specific locations along the chromosome toregulate genomic processes.
Histone variant H2A.Z decorates nucleosome at most promotersin yeast and mammalian cells. H2A.Z in corporation alters chromatin structure to modulate transcriptional responses. H2A.Z-H2B dimer deposition is catalyzed by chromatin remodelling complex SWR, a process accompanying with the removal of nucleosomal H2A-H2B dimer. SWR subunit Swc5, which is essential for the exchange process, has been implicated in H2A-H2B dimer removal. However, how Swc5 liberates the nucleosomal H2A-H2B to orchestrate nuclesome editing remains unclear.
A new study conducted by Dr. Zheng Zhou's group from the Institute of Biophysics of the Chinese Academy of Sciences and Dr. Ed Luk's group from Stony Brook University has revealed the molecular mechanism of Swc5 in regulating SWR-catalyzed histone exchange. This studyis published in Proceedings of the National Academy of Sciences of the United States of America on January 30th, 2020, with the title of "Role of a DEF/Y motif in histone H2A-H2B recognition and nucleosome editing".
In this study, researchers determined a 2.37angstrom crystal structure of Swc5 in complex with H2A-H2B, providing the structural basis for how Swc5 contributes to the directionality of the H2A-to-H2A.Z nucleosome editing process. The structure shows that Swc5 engages H2A-H2B using a tandem DEF/Y motif within its intrinsically disordered region, generating a unique "tridentate mode," which includes a core electronic interaction network and flanking hydrophobic interactions.
With the recombination of ITC assay and in vitro histone exchange experiment, researchers show that DEF/Y motif of Swc5 is required for H2A-H2B binding and optimal SWR function, whose mutation inhibits the nucleosome editing function of SWR in vitro. Moreover, in vivo crosslinking analysis shows that Swc5 DEF/Y interacts with histone in yeast cell, and the extent of this interaction is dependent on the remodeling ATPase of SWR, supporting a model in which Swc5 acts as a wedgebetween the nucleosomal DNA and H2A-H2B to promote H2A-H2B dimer eviction (Fig. 1).
Altogether, these findings lay the molecular basis of Swc5 in recognition of H2A-H2B and providecritical insight into how Swc5 facilitates SWR-catalyzed nucleosome editing.
The staff of the BL-19U1 beamlines at the Shanghai Synchrotron Radiation Facility and the Institute of Biophysics have provided technical support to this work.
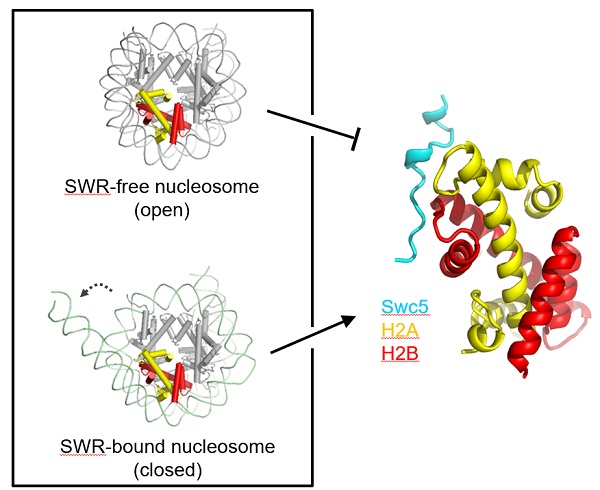
Swc5 recognizes H2A-H2B dimer (right panel) to facilliates SWR-catalyzed H2A removal (left panel)
(Image by Dr. ZHOU Zheng's lab)
The web link for this paper is https://doi.org/10.1073/pnas.1914313117
Contact: ZHOU Zheng
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889862
(Reported by Dr. ZHOU Zheng's group)

