New insights into the fusion of inner mitochondrial membranes by Mgm1
Prof. HU Junjie from the Institute of Biophysics of the Chinese Academy of Sciences and his collaborators reported structural and functional analysis of dynamin-like GTPase Mgm1. Their finding, published in PNAS on February 10, provides important insight into how inner mitochondrial membranes are fused.
In eukaryotic cells, mitochondria constantly fuse and divide, all of which are mediated by dynamin-like proteins (DLPs). Inner mitochondrial membrane (IMM) fusion requires optic atrophy 1 (OPA1) in mammals and mitochondrial genome maintenance 1 (Mgm1) in yeast.
Deletion or mutation of OPA1/Mgm1 in cells results in loss of mitochondrial DNA and mitochondrial fragmentation, and mutation of human OPA1 is linked to optic atrophy. Prof. HU's previous work demonstrated howsimilar GTPases mediate fusion of ER membranes and outer mitochondrial membranes, but it is unclear how IMM is fused.
The structure of budding yeast Mgm1 in a GDP-bound state was determined. It revealed an overall configuration that is very similar to scission DLP dynamin-1. In the crystal, Mgm1 forms a "head-to-tail" trimer, and each protomer also forms "back-to-back" dimer, resulting in a hexamer.
Mgm1 interacted with negatively charged lipids using at least two sites: the N-terminal GTPase (G) domain and the C-terminal lipid-interacting stalk (LIS). The curved shape of the molecule would allow membrane bending upon association.
Membrane-associated assembly of Mgm1 was found to stimulate GTP hydrolysis in a cardiolipin (an IMM-enriched lipid) dependent manner. Mutations in the "back-to-back" interface severely affected the stimulation but mutations in the "head-to-tail" interface did not.
Dimeric and trimeric interactions were confirmed in vitro using purified proteins. Structure-based mutagenesis was also performed in yeast cells. The functional analysis is consistent with a mechanism of Mgm1 forming "head-to-tail" and "back-to-back" assemblies.
These findings suggest that membrane targeting via the G domain and LIS facilitates the in cis assembly of Mgm1, potentially generating a highly curved membrane tip to allow inner membrane fusion. Our observations underscore the structural plasticity of dynamins and suggest a new mode of how they might mediate membrane fusion events.
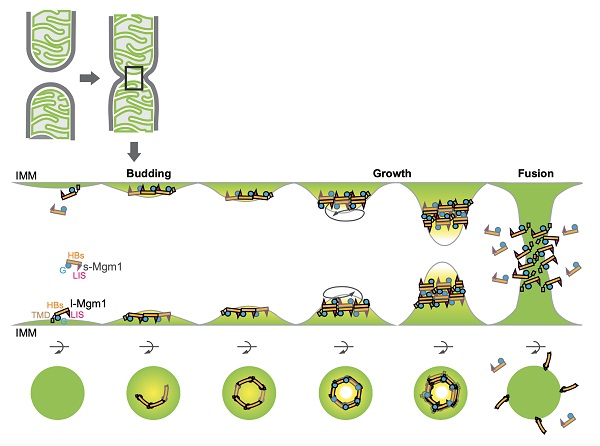
A model for Mgm1-mediated membrane fusion
(Image by Dr. Hu Junjie's lab)
The web link for this paper is https://www.pnas.org/content/early/2020/02/06/1919116117
Contact: HU Junjie
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889862
Email:huj@ibp.ac.cn
(Reported by Dr. Hu Junjie's group)

