The group of Prof. Sarah Perrett has made important progress in the study of the kinetic mechanism ofthe molecular chaperone Hsp70
Hsp70 is a coremolecular chaperone which participates in regulating protein folding, translocation and degradation and plays a key role in maintaining cellular proteostasis. Hsp70 consists of a nucleotide-binding domain and substrate-binding domain. Hsp70 binds and hydrolyzes ATP into ADP through its nucleotide-binding domain, regulating substratebinding and release by the substrate-binding domain through allosteric communication. Hsp70 cooperates with the cochaperone Hsp40, which stimulates the ATPase activity of Hsp70 and promotes the functional cycle and substrate refolding efficiency. Previous structural biology studies have resolved the full-length structure of Hsp70 in different nucleotide-bound states, but the interdomain conformational dynamics of Hsp70 as well as the mechanism by which Hsp40 regulates the intra- and intermolecular changes of Hsp70 are still largely unknown.
In this study, using singlemolecule fluorescence resonance energy transfer (FRET) techniques, we studied the interdomain conformational distribution of human stress-inducible Hsp70 (hHsp70) and the kinetics of conformational changes induced by nucleotide and the Hsp40 cochaperone Hdj1. We found that the conformations between and within the nucleotide- and substrate-binding domains show heterogeneity. The conformational distribution in the ATP-bound state can be induced by Hdj1 to form an "ADP-like" undocked conformation, which is an ATPase-stimulated state. Kinetic measurements indicate that Hdj1 binds to monomeric Hsp70 as the first step, then induces undocking of the two domains and closing of the substrate-binding cleft. Dimeric Hdj1 then facilitates dimerization of Hsp70 and formation of a heterotetrameric Hsp70-Hsp40 complex. Our results provide a kinetic view of the conformational cycle of Hsp70 and reveal the importance of the dynamic nature of Hsp70 for its function. This work also provides new insights for further elucidation of the differences between Hsp70s with high homology in sequence and structure, and the relationship between structure and function.
This study entitled "Kinetics of the conformational cycle of Hsp70 reveals the importance of the dynamic and heterogeneous nature of Hsp70 for its function" was published on-line in the Proceedings of the National Academy of Sciences on 20 March, 2020. Prof. Sarah Perrett is the corresponding author. Dr. WU Si, an associate professor in Prof. Sarah Perrett's group, Dr. HONG Liu, an associate professor at Tsinghua University, and WANG Yuqing, a PhD student in Prof. Sarah Perrett's group, are the co-first authors. The work was funded by the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
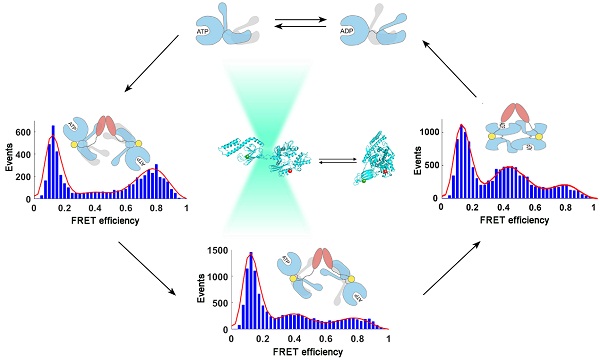
Legend: The conformational dynamics of human stress-inducible Hsp70 and its intra- and intermolecular changes induced by human Hsp40 Hdj1 revealed by single-molecule FRET.
(The image by Dr. Sarah Perrett's Lab)
Article link: https://www.pnas.org/content/early/2020/03/19/1914376117
Contact: Sarah Perrett
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889870
Email: sperrett@ibp.ac.cn
(Reported by Dr. Sarah Perrett's group)

