Integrated-omics of Deteriorating Pancreatic Islets Revealing the Chronological Order of T2D-related Molecular Events
Progressive reduction in β-cell mass and function comprises the core of the pathogenesis mechanism of type 2 diabetes (T2D) . In the UK Prospective Diabetes Study, researchers found that declining β-cell function was an early event during the course of T2D and was far advanced by the time diabetes was diagnosed. Though, many patients could achieve the glycemic goal (HbA1c < 7.0%) during a period of time with the help of certain medications, it couldn’t address the problem if β-cell function wasn’t improved. Recent genome-wide association (GWAS) and sequencing studies identified multiple risk variants for T2D, the majority of which appeared to have a primary role in β-cell function rather than an impact on insulin resistance, further highlighting the importance of β-cells in the pathogenesis of T2D.
The study entitled Temporal Transcriptomic and Proteomic Landscapes of Deteriorating Pancreatic Islets in Type 2 Diabetic Rats was published online in Diabetes on July 21, 2017. In this study, Prof. XU Tao’s group in Institute of Biophysics, Chinese Academy of Sciences generated integrated temporal transcriptomic and proteomic profiles of pancreatic islets in GK rats. This quantitative dataset provides a comprehensive picture of the mechanisms responsible for islet dysfunction and will allow researchers to identify potential interventions to prevent β-cell failure and deterioration in human T2D.
The Goto–Kakizaki (GK) rat is one of the best-characterized animal models of spontaneous T2D, shares many characteristics with human diabetic patients. Similar to human T2D, the core cause underlying hyperglycemia in GK rats is β-cell failure. Based on RNA Sequencing and tandem mass tag (TMT) based quantitative proteomics technology, Prof. XU Tao’s group carried out a large-scale analysis of gene and protein dynamics in pancreatic islets of GK rats at different stages of T2D. Combined transcriptome and proteome analysis revealed sufficient depth of coverage and quantitative accuracy to generate functional portraits of healthy and diseased pancreatic islets with unprecedented detail. Subsequent bioinformatics analysis in a time-course fashion revealed the chronological order of T2D-related molecular events during the deterioration of pancreatic islets. The omics dataset outlined the dynamics of molecular network during the deterioration of GK islets as two stages: the early stage (4-6 weeks) is characterized by anaerobic glycolysis, inflammation priming, and compensation for insulin synthesis, whereas the late stage (8-24 weeks) is characterized by inflammation amplification and compensation failure. Further time-course analysis revealed 5551 differentially expressed genes, a large portion of which have not been reported before. Moreover, researchers also found that if 4-week-old GK rats were treated with an antioxidant, the rats would exhibit both reduced random blood glucose and greater tolerance to high glucose, which indicated that Oxidative stress played an important role in the development of T2D at very early stage. In-depth exploration of this resource will aid in the discovery of potential diagnostic and therapeutic targets for human T2D. Furthermore the study also showed the power of system biology in the area of complex diseases. Methods of bioinformatics could help us to understand the mechanisms of diseases better.
Prof. XU Tao and Dr. WANG You are the corresponding authors. HOU Junjie, Associate Professor in the group of Prof. XU Tao, Joint PhD students LI Zonghong (Northeast Normal University) and ZHONG Wen(HuaZhong University of Science and Technology) are the co-first authors. This work was supported by grants from the National Key Basic Research Project of China, the Strategic Priority Research Program of the Chinese Academy of Sciences, the National Science Foundation of China, the National Key Basic Research Project of China.
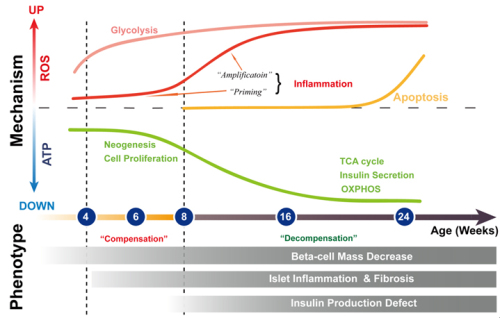
Figure. Time course of pancreatic islet deterioration in GK rats (Image by IBP)
Contact:
XU Tao
Principal Investigator
Institute of Biophysics
Email:xutao@ibp.ac.cn
Tel: 86-10-64888524
Fax:86-10-64888524

