The Composition of a Protein Aggregate Modulates the Specificity and Efficiency of Its Autophagic Degradation
Autophagy involves the engulfment of a portion of the cytosolic contents in a double-membrane structure, called the autophagosome, and its subsequent delivery to the lysosome for degradation. Many protein aggregates are selectively recognized and degraded by autophagy. Accumulation of protein aggregates is associated with a variety of human diseases. Whether the distinct composition of a protein aggregate affects its degradation efficiency remains largely unknown.
Recently, a study led by Prof. ZHANG Hong from the institute of Biophysics of the Chinese Academy of Sciences was reported in Autophagy on Aug. 14, 2017, entitled The composition of a protein aggregate modulates the specificity and efficiency of its autophagic degradation.
During C. elegans embryogenesis, two components of oocyte-derived P granules, PGL-1 and PGL-3 are degraded by autophagy in somatic cells. This process requires the self-oligomerized receptor protein SEPA-1 and the scaffold protein EPG-2. In this study, we found that depletion of PGL-1 or PGL-3 facilitates the degradation of SEPA-1 and EPG-2. Removal of EPG-2 is also promoted when SEPA-1 is absent. Depletion of PGL-1 or PGL-3 also renders the degradation of SEPA-1 independent of EPG-2. Over-expression of PGL granules or SQST-1 aggregates, which belong to different classes of aggregate and require distinct scaffold proteins for degradation, has no evident effect on the degradation of the other type. These results indicate that autophagic degradation efficiency is modulated by the composition of protein aggregates and is controlled at multiple levels.
This study was supported by the grants from the National Natural Science Foundation of China, the National Basic Research Program of China, and in part by an International Early Career Scientist Grant from the Howard Hughes Medical Institute.
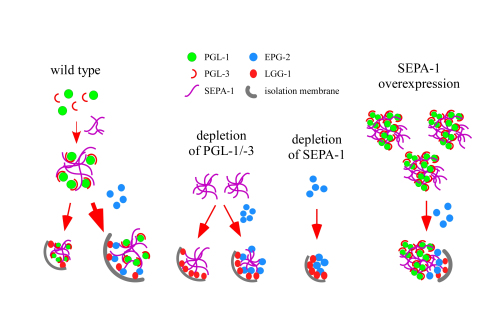
Figure: Model showing how the composition of a protein aggregate modulates the specificity and efficiency of its autophagic degradation (Image by IBP)
Contact:
ZHANG Hong
Institute of Biophysics
E-mail:hongzhang@sun5.ibp.ac.cn
Tel:86-10-64848238

