SIAH E3 Ubiquitin Ligase Regulation of Wnt/β-catenin Signaling Identified by IBP And NIBR Scientists
The Wnt/β-catenin signaling pathway plays essential roles during embryonic development and tissue homeostasis through controlling the stability of transcription cofactor β-catenin. In absence of Wnt ligands, β-catenin is associated with the β-catenin destruction complex that contains β-catenin, Axin, GSK3, and APC, phosphorylated by GSK3, and degraded by the ubiquitin proteasome pathway. In a Frizzled and Dishevelled-dependent manner, Wnt induces phosphorylation of its coreceptor, LRP6, and recruits Axin-containing β-catenin destruction complex to the plasma membrane to form the signalosome. Within the signalosome, GSK3 is inhibited by phospho-LRP6, which leads to destabilization of the β-catenin destruction complex and accumulation of β-catenin. Various mechanisms are evolved to control Wnt/β-catenin signaling output, and deregulation of the Wnt pathway is associated many diseases including cancer.
Through direct interaction with β-catenin, GSK3 and APC, the scaffolding protein Axin is responsible for the formation of the β-catenin destruction complex. The physical interaction between Axin and GSK3 is regulated. Association between Axin and GSK3 requires the GSK3 kinase activity. Wnt-induced phospho-LRP6 directly inhibits the kinase activity of GSK3 bound to Axin, which might be responsible for decreased Axin-GSK3 association upon Wnt treatment. The Axin-GSK3 interaction is also regulated by phosphorylation; CK1 and PP1 can reciprocally regulate the interaction between Axin and GSK3. Importantly, Axin is a concentration limiting factor for assembly of the β-catenin destruction complex, so mechanisms that control the protein level of Axin are expected to have major impacts on Wnt/β-catenin signaling. Indeed, stabilization of Axin using TNKS inhibitor strongly inhibits Wnt/β-catenin signaling. It has been known for many years that activation of Wnt or inhibition of GSK3 promotes degradation of Axin. Wnt-induced stabilization of β-catenin precedes Wnt-induced Axin degradation, so Axin degradation is not required for the initial Wnt-induced β-catenin stabilization. Despite of strong interest of studying Axin regulation, the molecular mechanism and biological relevance of Wnt-induced Axin degradation have been elusive.
Professor LIANG Dongcai and Associate Professor YAN Xiaoxue of the Institute of Biophysics (IBP) of the Chinese Academy of Sciences, in cooperation with Professor CONG Feng of Novartis Institutes for Biomedical Research and colleagues, identify SIAH1/2 (SIAH) as the E3 ligase mediating Wnt-induced Axin degradation. SIAH proteins promote ubiquitination and proteasomal degradation of Axin through interacting with a VxP motif in the GSK3 binding domain of Axin, and this function of SIAH is counteracted by GSK3 binding to Axin. Structural analysis reveals that the Axin segment responsible for SIAH binding is also involved in GSK3 binding, but adopts distinct conformations in Axin/SIAH and Axin/GSK3 complexes. Knockout of SIAH1 blocks Wnt-induced Axin ubiquitination and attenuates Wnt-induced β-catenin stabilization. Our data suggest that Wnt-induced dissociation of Axin/GSK3 complex allows SIAH to interact with Axin not associated with GSK3 and promote its degradation, and that SIAH-mediated Axin degradation represents an important feed-forward mechanism to achieve sustained Wnt/β-catenin signaling.
The research work, entitled The SIAH E3 ubiquitin ligases promote Wnt/β-catenin signaling through mediating Wnt-induced Axin degradation, was published as a cover article in the journal genes & development on June 1, 2017. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences and the National Science Foundation. And also was supported by Core Facilities for Protein Science at IBP, CAS, and at the National Center for Protein Science Shanghai (NCPSS).
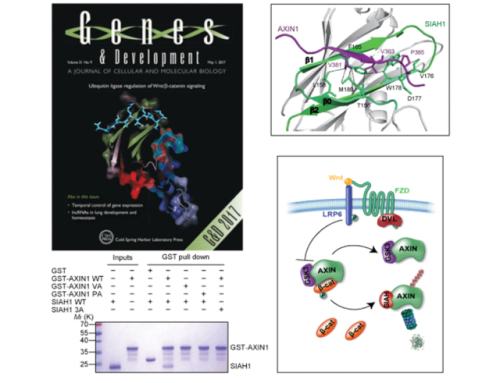
Figure: SIAH as the E3 ligase mediating Wnt-induced Axin degradation (Image by IBP)
Contact
YAN Xiaoxue
Institute of Biophysics, Chinese Academy of Sciences
Email: snow@ibp.ac.cn
CONG Feng
Novartis Institutes for Biomedical Research
E-mail: feng.cong@novartis.com

