A Bone Formation-related Ion Channel Visualized in Unprecedented Details
Calcium is the most abundant mineral in our body, a crucial element making up our bone and an essential supplement in our daily diets. Moreover, Ca2+ serves as a signaling messenger involved in all kinds of fundamental cellular activities such as muscle contraction, neurotransmitter release, cell growth and apoptosis. In eukaryotic cells, Ca2+ is mostly stored in the endoplasma reticulum (ER, an organelle found in the eukaryotic cells) or sarcoplasma reticulum (SR, a special type of ER found in muscle cells). During the signal transduction process induced by electrical or chemical stimuli, Ca2+ is released from the store through ryanodine receptor (RyR) or inositol-triphosphate receptor (IP3R). RyR and IP3R are two major types of Ca2+-release channels located on SR/ER membranes, respectively. Efficient release of Ca2+ from the store depends on not only RyR and IP3R, but also other ion channels on SR/ER membranes.
TRimeric Intracellular Cation (TRIC) channels are a family of newly identified ion channels on SR/ER membrane. They support the release of Ca2+ through RyR or IP3R and maintain the homeostasis of ions within the SR/ER lumen. TRIC channels have important physiological functions in the biological processes related to muscle contraction and bone formation. Animals lacking TRIC channels show symptoms of hypertension, heart diseases, respiratory defects and fragile bones. Two subtypes of TRIC channels, namely TRIC-A and TRIC-B, are present in human and other animals. Recent genetic studies highlighted the pivotal roles of TRIC-B protein in lung development and bone formation. For instance, brittle bone disease (also known as osteogenesis imperfecta/OI) is a human genetic disorder occurring ~1 in 20,000 individuals (according to NIH), and some OI patients were found to bear mutations in the TMEM38B gene encoding TRIC-B protein.
In a recent work published online in Nature journal, YANG et al. (led by Dr. LIU Zhenfeng at the Institute of Biophysics of Chinese Academy of Sciences) reported the long-awaited structures of TRIC-B channels (from Caenorhabditis elegans) for the first time (see the figure shown below). The structures were captured at two different conformational states and striking features about TRIC channels have been deciphered in great details. Unexpectedly, TRIC-B protein was found to bind a lipid molecule (named phosphatidylinositol 4,5-biphosphate, PIP2) specifically, and they forms a stable homotrimeric complex. Each monomer of TRIC channels contains an asymmetrical pore permeable to monovalent cations, and the three monomers cooperate with each other to achieve simultaneous opening. Remarkably, the endogenous PIP2 molecule mediates trimerization of TRIC channels, and also contributes directly to the formation of pore architecture. Furthermore, the TRIC-B channels exhibit two different conformations under Ca2+-bound and Ca2+-free states. The findings led to a mechanistic model explaining how TRIC channels respond to Ca2+ and voltage so as to become activated and open their gates for K+ or Na+ to flow through. These results provide a framework for further investigations on the molecular mechanism, physiological function, pathophysiological roles and pharmacological potentials of the TRIC channel family.
The work is entitled "Pore architecture of TRIC channels and insights into their gating mechanism", and published online by the NATURE journal on Oct 3, 2016. The research project is financially supported by the National 973 Program from Chinese Ministry of Science and Technology, the Strategic Priority Research Program and the "135" project of the Chinese Academy of Sciences (CAS). YANG Hanting and HU Miaohui, the two co-first authors with equal contributions, are graduate students from Liu lab. Dr. Guo Jianli Guo and Ms. OU Xiaomin performed electrophysiological experiments and data analysis in this research. Dr. CAI Tanxi (from YANG Fuquan lab at IBP) carried out mass spectrometry experiments on the lipid samples. The X-ray diffraction data were collected at the BL17U beamline of Shanghai Synchrotron Radiation Facility and BL1A, BL5A and NW12A beamlines of the Photon Factory. Dr. SUN Jianyuan's group at IBP provided the access to their electrophysiological devices used in this work.
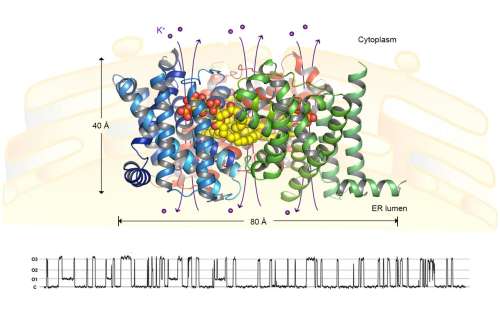
Figure legend A homotrimeric CeTRIC-B1 channel in complex with PIP2 lipid molecules. The protein subunits are presented as ribbon models colored in blue, green and red. The PIP2 lipid molecules are shown as yellow-and-red sphere models. The views are along the membrane plane. Below the structure, a trace of the electrical activities CeTRIC-B1 measured through the inside-out patch-clamp electrophysiology is shown. (Image provided by YANG Hanting and LIU Zhenfeng). A cartoon model of the endoplasma reticulum, where the TRIC channels are located, is shown in the background. (Image by IBP)
Contact:
LIU Zhenfeng, Ph.D.
Principal Investigator
National Laboratory of Biomacromolecules
Institute of Biophysics, Chinese Academy of Sciences
Email:

