Vici Syndrome Protein EPG5 Acts As a Tether to Determine Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes
Autophagy is an evolutionarily conserved lysosome-mediated degradation process. Autophagy in higher eukaryotes involves the formation of the double-membrane autophagosome and its subsequent fusion with endocytic vesicles and eventually with lysosomes, a process known as autophagosome maturation. The mechanism underlying specific fusion of autophagosomes with late endosomes/lysosomes remains largely unknown.
Recently, a study led by Prof. ZHANG Hong from the Institute of Biophysics (IBP) of the Chinese Academy of Sciences (CAS) was reported in Molecular Cell on Sep. 1, 2016, entitled "The Vici Syndrome Protein EPG5 Is a Rab7 Effector that Determines the Fusion Specificity of Autophagosomes with Late Endosomes/Lysosomes."
EPG5 was first identified as a metazoan-specific autophagy gene by genetic screens in C. elegans done by ZHANG’s group. EPG5 deficiency in C. elegans, medaka fish, mice and humans blocks the maturation of autophagosomes into degra-dative autolysosomes.
Recent human genetic studies revealed that human EPG5 causes Vici syndrome, a multisystem disorder with a wide range of clinical manifestations, including agenesis of the corpus callosum, myopathy and combined immunodeficiency.
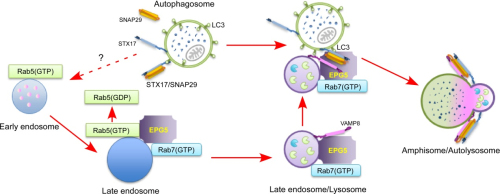
In their study, the researchers found that EPG5 acts as a tethering factor to mediate specific fusion of autophagosomes with late endosomes/lysosomes. EPG5 is recruited to late endosomes/lysosomes by direct interaction with Rab7 and VAMP7/8. EPG5 also binds to LC3/LGG-1 (mammalian and C. elegans Atg8 homolog, respectively) and to assembled STX17-SNAP29 Qabc SNARE complexes on autophagosomes.
EPG5 stabilizes and facilitates the assembly of STX17-SNAP29-VAMP7/8 trans-SNARE complexes, and promotes STX17-SNAP29-VAMP7-mediated fusion of reconstituted proteoliposomes. Loss of EPG5 activity causes abnormal fusion of autophagosomes with various endocytic vesicles and assembly of physiologically non-cognate SNAREs.
The study reveals that EPG5 is a Rab7 effector involved in autophagosome maturation, providing insight into the molecular mechanism underlying Vici syndrome.
This study was supported by grants from the National Natural Science Foundation of China, the National Basic Research Program of China, and in part by an International Early Career Scientist Grant from the Howard Hughes Medical Institute.
Model showing how EPG5 mediates fusion of autophagosomes with late endosomes/lysosomes (Image by IBP)
Contact:
ZHANG Hong
National Laboratory of Biomacromolecules, IBP
E-mail:hongzhang@sun5.ibp.ac.cn,Tel:010-64848238
Fax:86-10-64853925