Research Identifies the Membrane Pore in Pyroptotic Cell Death
A new study on mechanisms underlying membrane pore formation in pyroptosis sheds light on inflammatory responses and may help improve treatment of immunological diseases and septic shock.
The study, entitled “Pore-forming activity and structural autoinhibition of the gasdermin family,” was published on June 08 in Nature as an advanced online publication. Professor SHAO Feng, an investigator at the National Institute of Biological Sciences (NIBS) in Beijing and a guest investigator at the Institute of Biophysics of the Chinese Academy of Sciences, led the research as a collaboration with Professor WANG Da-Cheng’s group at the Institute of Biophysics of the Chinese Academy of Sciences.
Pyroptosis is a kind of programmed necrosis manifested by cell swelling and plasma membrane lysis, resulting in release of cytosolic contents and strong inflammation. Pyroptosis plays crucial roles in innate defense against infections and endogenous dangers. Excessive pyroptosis causes many inflammatory and immunological diseases including septic shock.
Pyroptosis is induced by two types of inflammatory caspases, caspase-1 and caspase-4/5/11. Caspase-1 was the first caspase identified and was shown to cause cell death in the early 1990s (misclassified as apoptosis then). Caspase-1 is activated by the inflammasome complex upon sensing microbial infection, representing a critical cytosolic innate defense. Human caspase-4/5 and mouse caspase-11 are activated by direct binding to cytosolic LPS (also known as endotoxin), playing a key role in bacteria-induced septic shock. In a breaking study published last year, the Shao group identifies a novel GSDMD protein whose cleavage by all inflammatory caspases is required and sufficient for the onset of pyroptosis.
GSDMD belongs to an unknown-function gasdermin family, which also includes GSDMA, GSDMB, GSDMC, DFNA5 and DFNB59. Genetic mutations in human DFNA5 and mouse Gsdma3 cause human nonsyndromic hearing impairment and mouse alopecia and skin inflammation, respectively. GSDMB polymorphism is associated with early childhood asthma.
The study in question discovers that the N-terminal Gasdermin-N domain conserved in the entire gasdermin family harbors pore-forming activity, which causes membrane disruption and the lytic pyroptotic cell death.
In this research, scientists first found that Gasdermin-N domains from nearly all family members harbored pyroptosis-inducing activity. Except for GSDMD, other gasdermins are not sensitive to cleavage by inflammatory caspases. Interestingly, Gasdermin-N domains also exhibited cytotoxicity when expressed in bacteria. This hints that Gasdermin-N may have membrane-disrupting activity and therefore kill both bacteria and mammalian cells. To test this hypothesis, the researchers prepared active GSDMD, GSDMA and GSDMA3, and showed that the three gasdermin proteins could specifically bind to two membrane lipids, phosphoinositides and cardiolipin. This property correlates well the cytotoxicity in mammalian and bacterial cells as phosphatidylinositol-4,5-bisphosphate and cardiolipin are major lipids in mammalian cell plasma membrane and bacterial membrane, respectively. Further biochemical and immunofluorescence imaging analyses showed that activated Gasdermin-N domain moved from the cytosol to plasma membrane during pyroptosis, following which membrane bubbles appeared accompanied by cell swelling.
Researchers further found that Gasdermin-N could efficiently and specifically induce leakage of phosphoinositide- or cardiolipin-containing liposomes. When liposomes encapsulated with different-size dextrans were assayed, it was noted that items with diameters of 10 nm or less could pass through the broken liposome, suggesting that Gasdermin-N may form regular membrane pores. Biochemical assay confirmed the oligomerization of Gasdermin-N upon liposome binding or translocation onto the plasma membrane during pyroptosis.
Using the negative-stain electron microscopy technology, researchers were able to observe that gasdermin-N domain formed multiple pores of “Swiss cheese”-like shape on membranes made of artificial or natural phospholipid mixtures. Most gasdermin pores had an inner diameter of 10–14 nm with a 16-fold symmetry, suggesting a 16-mer symmetric pore complex formed by the Gasdermin-N domain. The researchers also determined the 1.90 ? crystal structure of GSDMA3, which revealed unique structural feature of gasdermin-N as a novel type of pore-forming protein as well as the detailed autoinhibitory interactions. Structure-guided mutation analyses further confirmed that the liposome-binding and pore-forming activities of gasdermin-N are the molecular basis for its pyroptosis-inducing activity.
The study demonstrates that GSDMD protein is the executioner in inflammatory caspase-mediated pyroptosis, and more importantly reveals that the gasdermin family has a pore-forming membrane-disrupting activity. This finding re-defines the concept of pyroptosis as gasdermin-mediated programmed necrosis, opening a new field in programmed necrosis. The study should guide GSDMD-based drug development for some autoinflammatory diseases and septic shock, and also paves the way for future studies to elucidate the functional mechanism of other gasdermins in immunity or other physiological processes.
This research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the 973 National High-Technology Projects Program, the Beijing Scholar Program of the Beijing Municipal Government, the China National Science Foundation and the Howard Hughes Medical Institute in the United States.
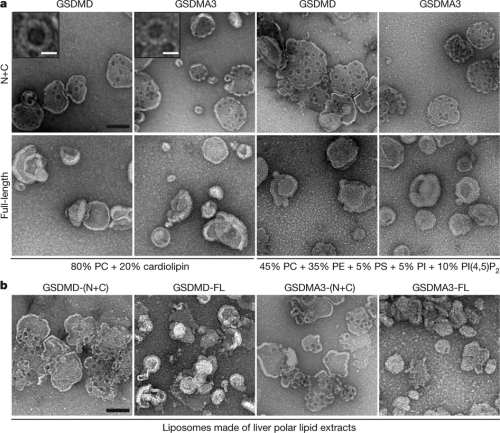
Liposomes made of liver polar lipid extracts (Image by SHAO Feng)
Contact
SHAO Feng
National Institute of Biological Sciences
guest investigator at the Institute of Biophysics
Email: shaofeng@nibs.ac.cn
WANG Da-Cheng
Institute of Biophysics

