Collaboration between IBP and the University of Cambridge Produces Enzymatically Active Microgels from Self-Assembling Amyloid Fibrils
Amyloid fibrils are highly ordered protein or peptide fibrillar aggregates associated in nature with both neurodegenerative diseases and with functional roles. The extraordinary stability and tunable self-assembly of amyloid fibrils has motivated their exploration as potential nanomaterials.
In a collaboration between the group of Prof. Sarah Perrett at the Chinese Academy of Sciences Institute of Biophysics (IBP) and the group of Prof. Tuomas Knowles in the Chemistry Department at the University of Cambridge, microfluidic techniques were used to generate enzymatically active microgels that are stabilized by amyloid nanofibrils.
A microgel is a three-dimensional colloidal network of micron-scale size and can be applied in biomedical applications, such as drug delivery, microsensor biomaterials. To date, most microgels have been generated using synthetic molecules, with the polymerization reaction often requiring non-biocompatible conditions or reagents, such as extreme pH, temperature, reactive chemicals or UV radiation. In contrast, microgels composed from naturally occurring polymers such as proteins or peptides are more likely to be biocompatible and biodegradable. Protein-based materials offer the additional advantage that novel functions can be directly incorporated via gene fusion producing a single chimeric polypeptide that will both self-assemble and display the desired activity.
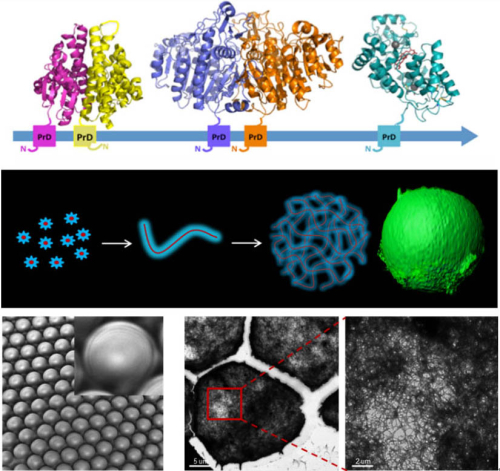
The yeast prion protein, Ure2, has been explored previously as a nanoscaffold for displaying and immobilizing enzymes via genetic fusion (Zhou et al. & Perrett (2014) ChemCatChem 6, 1961-1968. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4413355/). In the current study, a chimera containing the prion domain of Ure2 fused to the enzyme alkaline phosphatase was used as the material for microgel formation. The ability of the chimeric protein to self-assemble under mild conditions enables the formation of catalytic microgels whilst maintaining the integrity of the encapsulated enzyme. The enzymatically-active microgel particles show robust material properties and their porous architecture allows diffusion in and out of reactants and products. In combination with microfluidic droplet trapping approaches, enzymatically-active microgels illustrate the potential of self-assembling materials for enzyme immobilization and recycling, and for biological flow-chemistry. These design principles can be adopted to create countless other bioactive amyloid-based materials with diverse functions.
This study entitled “Enzymatically Active Microgels from Self-Assembling Protein Nanofibrils for Microflow Chemistry” was published on-line in ACS Nano on June 1, 2015. The experimental work was primarily carried out by Xiaoming Zhou, a PhD student in the group of Prof. Sarah Perrett at IBP, and Ulyana Shimanovich, a post-doc in the groups of Prof. Chris Dobson, FRS, and Prof. Tuomas Knowles at the University of Cambridge.
The work was funded by the National Natural Science Foundation of China and the 973 Program of the Ministry of Science and Technology.
Legend: The Ure2 prion domain as a scaffold for enzyme immobilization and microgel formation.
(Figure adapted from Zhou et al. & Perrett (2014) ChemCatChem 6,1961-1968 and Zhou et al. & Perrett (2015) ACS Nano published on-line 1 June 2015.)
CONTACT:
Sarah Perrett
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889870
Email: sperrett@ibp.ac.cn