New insights into the regulatory mechanism of histone variants to gene transcription
Dr. Guohong Li’s group at the Institute of Biophysics, CAS, published an article on Genes & Development in October 2013, entitled “H3.3 actively marks enhancers and primes gene transcription via opening higher-ordered chromatin”. The article reports the biochemical mechanisms of two important histone variants H3.3 and H2A.Z on regulating chromatin higher-ordered structure and gene transcriptional activity.
Histone variants are non-allelic forms of canonical histones. The incorporation of histone variants may change the local and global structures of chromatin and create architecturally distinct chromatin states that perform diverse functions. The variants H2A.Z and H3.3, both of which are highly conserved evolutionarily, have been proposed to play crucial and specific roles in the regulation of chromatin dynamics and transcription. Previous study showed that histone variant H2A.Z can form a kind of more compact chromatin. Interestingly, histone variant H3.3, with highly similar amino acid sequence to canonical H3, can play a distinct role in the process of transcriptional regulation and cell development. But the functional mechanism is still poorly understood.
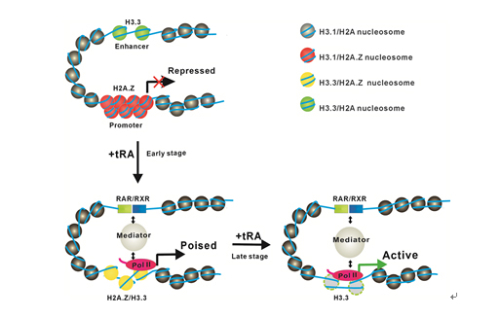
Dr. Li's group found that histone variant H3.3 greatly impairs the folding of chromatin fibers and counteracts H2A.Z-mediated compaction in vitro. And through in vitro transcription assay, they found that H3.3 highly antagonizes the inhibition of H2A.Z on chromatin transcriptional activity. Furthermore, they demonstrated that in vivo H3.3 actively marks enhancers and determines transcriptional potential of retinoid acid (RA)-regulated genes via creating an open chromatin signature that enables the binding of RAR/RXR. Knock-down of H3.3 in mouse embryonic stem cells (mESCs) greatly impaired the localization of H2A.Z, RAR and TBP to promoter regions. The H3.3-dependent recruitment of H2A.Z on promoter regions resulted in compaction of chromatin to poise transcription; while RA induction results in the incorporation of H3.3 on promoter regions to activate transcription via counteracting H2A.Z-mediated chromatin compaction. Their work provides key insights into the mechanism of how histone variants H3.3 and H2A.Z function together to regulate gene transcription via the modulation of chromatin dynamics over the enhancer and promoter regions.
Figure 1: A model for the dynamic regulation of H2A.Z and H3.3 on gene activation
This work was supported by grants from the Ministry of Science and Technology of China, the Natural Science Foundation of China, Chinese Academy of Sciences Strategic Priority Research Program and the Key Research Program. It was a cooperative achievement among scientists from the Institute of Biophysics, the Institute of Physics and Beijing Institute of Genomics in Chinese Academy of Sciences. Detailed information can be found at the website:
http://genesdev.cshlp.org/content/early/2013/09/22/gad.222174.113