Structural study reveals a novel cell membrane remodeling mechanism
Cell membrane remodeling is critical for many aspects of cellular function, including biogenesis of organelles and transport carriers, cell motility, and cytokinesis, the division process of the cytoplasm of a eukaryotic cell.
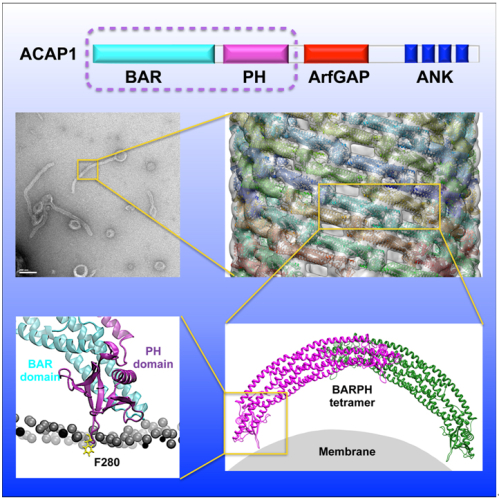
It is believed that the Bin-Amphiphysin-Rvs (BAR) domain-containing proteins areimportant membrane remodelers. They undergo dimerization to produce a curved protein structure, which superimposes onto membrane through electrostatic interactions to sense and impart membrane curvature.
Currently, two general mechanisms are known for how the BAR domains induce membrane curvature. One mechanism is based on scaffolding, which is initiated through dimerization that results in a banana-like structure. This structure imposes curvature on membrane through positively charged patches on the concave surface of BAR proteins that interact with the negatively charged surface of membrane through electrostatic interactions, and then induces membrane curvature. The other general mechanism involves membrane insertion, which typically involves an amphipathic helix, such as that seen for the N-BAR-containing endophilin. Curiously, some BAR domains have been found to require an additional neighboring PH domain to bind membrane and induce curvature,but why the PH domain is required has been unclear.
Professor SUN Fei at the Institute of Biophysics, Chinese Academy of Sciences and his colleagues recently discovered a cell membraneremodeling mechanism different from theknown ones. In collaboration with Victor Hsu’s group from Harvard Medical Schooland Jun Fan’s group from City University of Hong Kong, SUN’s group had focused their study on BAR-PH domain of ACAP1(ArfGAP with coiled coil, ANK repeat and PH) andfound that BAR-PH can induce membrane curvature.
By utilizinga combinationof various technologies from Cryo-Electron Microscopy, X-ray crystallography, molecular dynamics, biochemistry, to cell biology methods, they have elucidated in molecular detail how ACAP1 remodels cell membrane through its role as a coat component and promotes the generation of transport carrier in endocytic recycling. Theyidentified that positively charged patches in the PH domain can engage phosphorylatedphosphoinositides of the membrane bilayer, and thereby allowing the key loop structure in this domain to be oriented properly for membrane insertion through a critical bulky hydrophobic residue (F280) to induce membrane curvature, while BAR domain in ACAP1 plays more of a supportive role in assisting the PH domain to bind and bend membrane and is organized into higher-order structure on tubulated membrane by lateral interactions with additional ‘end-to-arch’ interactions. Results from their Cryo-EM study provided the direct evidence for the proposed mechanism, while their biochemistry results were further confirmed by molecular simulation.
It is notable that, besides ACAP1, multiple other proteins are known to possess tandem BAR-PH domains. These include a number of ArfGAPs, as well as APPLs, and also sorting nexins that possess a PX domain that is highly similar to the PH domain. The new insights regarding the PH domain in ACAP1 provides researchers with a clearer picture how these molecules act in membrane remodeling in membrane traffic pathway.
An article entitled “A PH domain in ACAP1 possesses key features of the BAR domain in promoting membrane curvature” introduced this work. It was published in the latest issue of Developmental Cell.
Figure:Molecular details on ACAP1 in membrane remodeling.Figuresin the amplified sequence: Negative-stain electron microscopy visualizing liposomes incubated with BAR-PH;CryoEM map of a liposomal tubule coated with BAR-PH; Structural model of BAR-PHhelical assembly on liposomal tubule; final simulated model of PH domain engaging the membrane.
Source: IBP, CAS