IBP scientists elucidated the role of protein N-terminal acetylation in nucleosome binding
Nucleosome is the basic unit of eukaryotic chromatin. Structural and functional studies of assembly, modification and recognition of the nucleosome are important aspects of epigenetic research. Despite its importance, solving the atomic resolution crystal structure of nucleosome-protein complexes poses great challenge to crystallographers because of the complexities of their interaction.
In Saccharomyces cerevisiae, silencing of the mating-type (HM) and telomeric loci requires the set of trans-acting Silent Information Regulators (Sir). Sir3 is one of the Sir proteins that interacts with the nucleosome through its N-terminal BAH domain. Acetylation of the Sir3 N terminus is important for transcriptional silencing. This covalent modification promotes the binding of the Sir3 BAH domain to the nucleosome, but a mechanistic understanding of this phenomenon is lacking.
Dr. XU Rui-Ming’s lab at the Institute of Biophysics, Chinese Academy of Sciences is specialized in studying epigenetic mechanisms. His group conducted structural and functional studies of the catalytic mechanisms and substrate specificities of histone modification enzymes, recognitions mechanisms of histone readers, structure and function of histone chaperons and variants, as well as nucleosome and protein interactions.
In this study, Dr. Xu’s group focused on the role of Nα-acetylation of the Sir3 BAH domain in nucleosome binding. They used baculovirus-infected insect cells to produce Nα-acetylated Sir3 BAH domain (acSir3BAH), and measured the binding affinity to NCP by ITC. The result showed that Sir3 N-terminal acetylation increased the NCP binding affinity by ~30 fold. By X-ray crystallography, they solved the structures of the acSir3BAH in complex with yeast Nucleosome. The structural work and accompanying mutagenesis and biochemical analyses showed that the acetylated N terminus of Sir3 does not interact with the nucleosome directly. Instead, it stabilizes a loop in the BAH domain in a conformation for productive interaction with the nucleosome.
Most eukaryotic proteins are acetylated at the N terminus, but the biological function of this com-mon modification in eukaryotes is diverse and not well understood. The present study reveals a novel function of protein N-terminal acetylation, and this result is helpful for understanding the structure and function of the vast eukaryotic N-terminal acetylome.
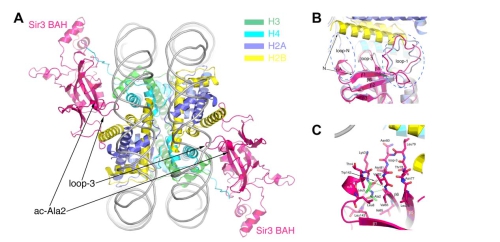
Figure (A) Overall structure of Sir3BAH in complex with yeast nucleosome. (B) Structural differences between the NCP-bound acetylated and unacetylated Sir3 BAH domains. (C) Intra- and intermolecular interactions of the acetylated N terminus and loop 3 of the Sir3 BAH domain.

