A Chemical Approach for Global Protein Knockdown from Mice to Non-human Primates
A research article entitled "A Chemical Approach for Global Protein Knockdown from Mice to Non-human Primates" was published online by Cell Discovery on February 5, 2019. In this paper, a rapid and reversible knockdown of FKBP12 protein in animals was achieved by chemical design of protein-degrading molecules (PROTACs), and rapid knockdown of protein in rhesus monkeys was successfully achieved for the first time. This paper was completed by cooperation of Guangju Ji's group from Institute of Biophysics, Chinese Academy of Sciences, Yu Rao’s group from Tsinghua University, and Xiuqin Zhang’s group from Peking University.
Traditionally, Loss of gene function studies have mainly been achieved through genetic modifications, such as RNA interference, transcription activator-like effector nucleases, recombination-based gene knock-out, clustered regularly interspaced short palindromic repeats (CRISPR-Cas9), and mediated genome editing. However, it remains challenging to apply these strategies in large animals, particularly in non-human primates. Besides, these approaches have failed to in a certain degree to achieve acute and reversible changes of gene function. Furthermore, the complications of potential genetic compensation and/or spontaneous mutations arising in gene-knockout models may lead to misinterpretations.
Proteolysis-targeting chimeras (PROTACs) contain a specific ligand for a target protein of interest that is connected to a ligand for an E3 ubiquitin ligase via a linker. FKBP12 protein is widely expressed in mammals. Through binding the Ca2+- release channel (ryanodine receptor), FKBP12 regulates Ca2+ signaling to carry out important functions, particularly in the heart. The global knockdown of FKBP12 via prevalent approaches is embryonic-lethal due to severe developmental heart defects such as ventricular septal defects.
In this work, authors developed a chemical approach for the global knockdown of targeted proteins using PROTACs. It is a novel, fast, and effective method for generating protein depletion animal models such as mice, rats, pigs, and rhesus monkeys. Furthermore, this strategy can also achieve conditional protein knockdown in the brain via i.c.v. administration. When the PROTAC probe is administered orally, this approach is also very effective. After withdrawal of the PROTAC, the FKBP12 protein level recovers in a certain period of time. The models are suitable for self-controlled biomedical studies. Mice and rhesus monkeys with FKBP12 knockdown via the chemical strategy exhibited Ca2+-leakage and increased Ca2+ sparks in cardiomyocytes, and cardiac dysfunctions. Thus, these models may be developed for cardiac drug screening. Additionally, this method can also be applied to other targets such as BTK. It may be a challenge to develop PROTAC for those targeted proteins without well-documented specific binders. Thus, PROTACs hold the promise to be employed as a complementary approach for currently available tools in a quick, controllable and reversible way for both in vitro and in vivo studies.
Commenting on this work, Wenyi Wei,from Harvard Medical School,in Cell Research says: “This strategy not only provides a powerful tool for protein function studies in vivo, but also highlights the potential of using PROTAC in future human cancer therapies”. Although FDA has approved the first oral PROTAC (arv-110) into a phase I clinical study at present, the PROTAC system research and animal level of preclinical research, especially large animals is lacking. Therefore, this work has realized the systematic exploration of PROTAC technology from mice, pigs to rhesus monkeys, filled in the blank of PROTAC technology preclinical research, and provided guidance for its application in clinical treatment of human diseases. As a laying solid foundation, it provides a new, efficient, rapid, reversible and low-cost tool for the study of protein function in vivo.
This work was supported by the National Natural Science Foundation of China, National Major Scientific and Technological Special Project, National Key R&D Program of China, the National Natural Science Foundation of China, the Drug Innovation Major Project, and Beijing Municipal Science & Technology Commission.
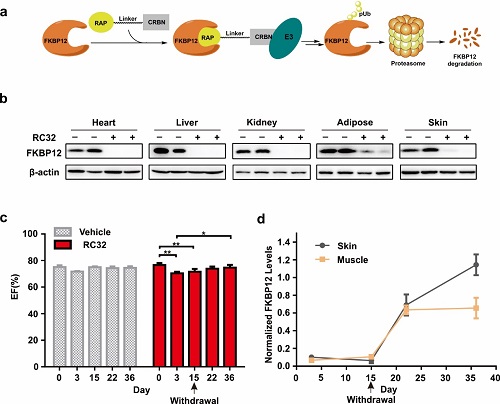
Figure: Protein degradation and recovery in rhesus monkeys, and changes in cardiac function by RC32.
(Imaged by Dr: Ji Guangju’s group)
Article link: https://www.nature.com/articles/s41421-018-0079-1
https://www.nature.com/articles/s41422-019-0144-9
Contact: Guangju Ji, Ph.D.
Principal Investigator
Institute of Biophysics Key Laboratory of Interdisciplinary Research
Institute of biophysics, Chinese Academy of Sciences
Beijing100101, China
Tel: (86)-10-64846720
Email: gj28@ibp.ac.cn
(Reported by Dr. Ji Guangju’s group)

