A new proof-of-concept study on targeting the oxidative protein folding pathway to suppress cervical cancer progression
Cervical cancer is one of the most common malignancies and a prominent cause of cancer-related deaths among women. Although concomitant chemoradiotherapy has been recommended as the standard treatment for cervical cancer, the5-year survival rate of these patients still needs to be improved. Therefore, new therapeutic targets are urgently needed.
Due to uncontrolled proliferation and growth, cancer cells require a great capacity for synthesis and folding of many cytokines and membrane receptors. Biosynthesis of all membrane and secretary proteins, which typically contain disulfide bonds, is performed in the endoplasmic reticulum (ER) lumen and occurs through the secretory pathway. ER oxidoreductin-1α (Ero1α) is a sulfhydryl oxidase located in the ER, which utilizes oxygen to oxidize protein disulfide isomerase (PDI) with hydrogen peroxide (H2O2) as a byproduct; oxidized PDI catalyzes the formation of disulfides in reduced substrates. Ero1α and PDI constitute the most pivotal oxidative protein folding pathway in the ER.
A study published in the recent issue of EBioMedicine by lead author Dr. Lei Wang and co-authors from the Institute of Biophysics, Chinese academy of sciences and Wenzhou Medical University, have succeeded in establishing a link between the oxidative protein folding pathway and cervical cancer, and provided more insights into the underlying mechanisms.
The researchers reported that elevated expression of Ero1α correlated with poor prognosis in human cervical cancer. Knockout of ERO1A decreased the growth, migration and tumorigenesis of HeLa cervical cancer cells in vitro and in vivo, through downregulation of the H2O2-dependent epithelial-mesenchymal transition. Mechanistic investigations showed that the conserved Valine (Val) 101 in the outer active site-containing loop of Ero1α was critical for the Ero1α-PDI complex formation and the oxidase activity of Ero1α. Val101 of Ero1α was specifically involved in the recognition of the catalytic domain of PDI. Mutation of Val101 resulted in a reduced ER redox state, retarded oxidative protein folding and decreased H2O2 level in the ER of HeLa cells, and further impaired cell migration, invasion, and tumor growth.
Overall, this study highlighted the critical role of oxidative protein folding in cervical cancer progression. Given that Ero1α and PDI proteins are upregulated in many types of cancers, the strategy of disruption of Ero1α-PDI interaction may be applied not only in cervical cancer but also in many other cancers.
Article link: https://www.ebiomedicine.com/article/S2352-3964(19)30119-7/abstract
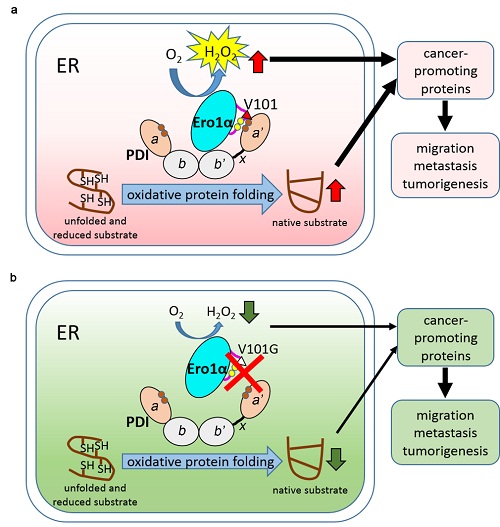
Figure. Targeting the functional interplay between Ero1α and PDIsuppresses the progression of cervical cancer
(Imaged by Dr: Wang Zhizhen’s group)
Contact: Wang Lei, Ph.D.
Associate Professor
Institute of biophysics, Chinese Academy of Sciences
Beijing100101, China
Tel: (86)-10-64888501
Email: wanglei@ibp.ac.cn
(Reported by Dr. Wang Zhizhen’s group)

