Development of nanoparticles as drug carrier with tumor-target therapy
A research entitled “Stimuli-Responsive Nanocarrier for Co-delivery of MiR-31 and Doxorubicin To Suppress High MtEF4 Cancer” was published online in the ACS Applied Materials & Interfaces on June 13th, 2018. The breakthrough is achieved through continuous and persistent efforts by QIN Yan group after their previous works on the mtEF4 (Cancer Research, 2018).
Gene interference-based therapeutics represents a fascinating challenge and show enormous potential for cancer treatment, in which microRNA is used to correct abnormal gene. On the basis of the above, we introduced microRNA-31 to bind to 3′-untranslated region of mtEF4, resulting in the downregulation of its messenger RNA and protein to trigger cancer cells apoptosis through mitochondria-related pathway. To achieve better therapeutic effect, a mesoporous silica nanoparticle-based controlled Nano platform had been developed. This system was fabricated by conjugation of microRNA-31 onto doxorubicin-loaded mesoporous silica nanoparticles with a poly(ethylene mine) / hyaluronic acid coating, and drug release was triggered by acidic environment of tumors. By feat of surface functionalization and tumor-specific conjugation to nanoparticles, our drug delivery system could promote intracellular accumulation of drugs via the active transport at tumor site. More importantly, microRNA-31 not only directly targeted to mtEF4 to promote cell’s death, but had synergistic effects when used in combination with doxorubicin, and achieved excellent super additive effects. As such, our research might provide new insights toward detecting high mtEF4 cancer and exploiting highly effective anticancer drugs.
Here, we developed an assembled delivery platform for co-delivery of microRNA and chemotherapy, which was easier to construct and modulate the size for cellular uptake. More importantly, this work achieved the synergistic effect of gene regulation and chemotherapy by introducing miR-31 and DOX to high mtEF4 cancer cells. Especially, the co-delivery of miR-31 with DOX using this platform inhibited cancer cell’s growth efficiently than delivering miR-31 or DOX alone. Next, we will be centered on the action mechanism between mtEF4 and antitumor drugs, which might provide a new idea for the further study of drug resistance.
QIN Yan group from Institute of Biophysics (IBP), Chinese Academy of Science and Wen Yongqiang Group from University of Science & Technology Beijing (USTB) are the corresponding authors. WANG Fang (USTB) and ZHANG Lingyun (IBP) are co-first authors. This work was supported by the foundations from the Chinese Ministry of Science and Technology, the National Natural Science Foundation and Key Projects of the Chinese Academy of Sciences.
Article link: https://pubs.acs.org/doi/pdfplus/10.1021/acsami.8b07698
Gene interference-based therapeutics represents a fascinating challenge and show enormous potential for cancer treatment, in which microRNA is used to correct abnormal gene. On the basis of the above, we introduced microRNA-31 to bind to 3′-untranslated region of mtEF4, resulting in the downregulation of its messenger RNA and protein to trigger cancer cells apoptosis through mitochondria-related pathway. To achieve better therapeutic effect, a mesoporous silica nanoparticle-based controlled Nano platform had been developed. This system was fabricated by conjugation of microRNA-31 onto doxorubicin-loaded mesoporous silica nanoparticles with a poly(ethylene mine) / hyaluronic acid coating, and drug release was triggered by acidic environment of tumors. By feat of surface functionalization and tumor-specific conjugation to nanoparticles, our drug delivery system could promote intracellular accumulation of drugs via the active transport at tumor site. More importantly, microRNA-31 not only directly targeted to mtEF4 to promote cell’s death, but had synergistic effects when used in combination with doxorubicin, and achieved excellent super additive effects. As such, our research might provide new insights toward detecting high mtEF4 cancer and exploiting highly effective anticancer drugs.
Here, we developed an assembled delivery platform for co-delivery of microRNA and chemotherapy, which was easier to construct and modulate the size for cellular uptake. More importantly, this work achieved the synergistic effect of gene regulation and chemotherapy by introducing miR-31 and DOX to high mtEF4 cancer cells. Especially, the co-delivery of miR-31 with DOX using this platform inhibited cancer cell’s growth efficiently than delivering miR-31 or DOX alone. Next, we will be centered on the action mechanism between mtEF4 and antitumor drugs, which might provide a new idea for the further study of drug resistance.
QIN Yan group from Institute of Biophysics (IBP), Chinese Academy of Science and Wen Yongqiang Group from University of Science & Technology Beijing (USTB) are the corresponding authors. WANG Fang (USTB) and ZHANG Lingyun (IBP) are co-first authors. This work was supported by the foundations from the Chinese Ministry of Science and Technology, the National Natural Science Foundation and Key Projects of the Chinese Academy of Sciences.
Article link: https://pubs.acs.org/doi/pdfplus/10.1021/acsami.8b07698
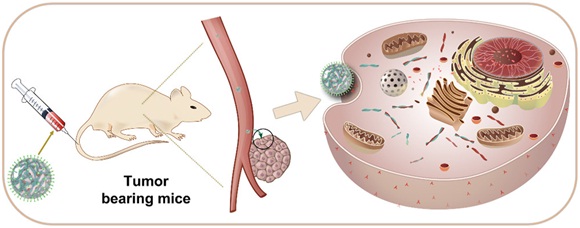
Schematic representation for construction of MSN/DOX/miR-31/PEI/HA (abbreviated as complex) and the mechanism of tumor-targeted combined therapy.
Contact: QIN Yan
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China

