Progress of Gene Encoded difluorotyrosine on Membrane Protein and Application of Gene Code Expansion in studying of Enzyme Catalysis Mechanism
Two research articles titled “Cleavage of a carbon-fluorine bond by an engineered cysteine dioxygenase” and “Allosteric mechanisms underlie GPCR signaling to SH3-domain proteins through arrestin” are published online on Nature chemical biology on June 25th and Aug 17th 2018. The work found that C-F bonds could be cleaved catalyzed by cysteine dioxygenase by using genetic code extension techniques. New progress was advanced by genetic code expansion technology in GPCR signal transduction research.
Fluorine is one of the most fundamental elements of life and widely used by medicinal chemists to treat cancer and in antibiotics, anti-depressants, steroids and other drugs. Cysteine dioxygenase (CDO) regulate the body’s thiol levels. When there’s too much thiol in the body, CDO develop catalytic amplifiers to quickly remove thiol from the body. Using the genetic code expantion, the researchers substituted CDO’s tyrosine with a laboratory-synthesized, double-fluorinated tyrosine. This created two very strong carbon-fluorine bonds in CDO where there would have been two much weaker carbon-hydrogen bonds and should have made it more difficult for the enzyme to break those carbon-fluorine bonds and produce its catalytic amplifier. High-precision mass spectrometry, NMR spectroscopy and X-ray crystallographic structural determinations revealed that CDO was still able to break its carbon-fluorine bonds to generate its full catalytic assembly – despite the fluorine substitution. To study the oxidizing power of the thiols, the researchers then performed cutting-edge techniques such as high-precision mass spectrometry, NMR spectroscopy and X-ray crystallographic structural determinations. They observed that CDO was still able to break its carbon-fluorine bonds to generate its full catalytic assembly - despite the fluorine substitution. Graduated student Jiasong Li is the first author of this waor.
GPCR (G-protein coupled receptor) is one of the most important drug targets and is the target of approximately 30% marketed clinically drugs nowadays. Despite its extremely important functions, the mechanisms beneath its signal transduction pathway have not been fully elucidated. In this study, with the help of genetic code expansion technique and 19F-NMR spectroscopy, researchers have revealed the mechanisms how arrestin mediating the signal transduction from 800 GPCRs to approximately 300 SH3 domain-containing proteins (SH3-CPs). The researchers found that different PRs (proline regions) in arrestin are docking sites in connecting different receptors to downstream SH3-CPs, and the conformational states between arretin and SH3-CPs are regulated through an allosteric mechanism. Same allosteric mechanism can be seen when receptor–arrestin complex promoting SRC kinase activity from in vitro reconstitution experiments, fluorescence quenching and BRET results. These results provide direct experimental evidence that allosteric regulation is a crucial mechanism underlying arrestin-mediated GPCR functions on at least two levels. The first level lies in the distinct, functionally related conformational states of arrestin directed by phosphorylated GPCR barcodes. Allosteric coupling between selective receptor-phosphosite binding and the specific conformational states of three PRs located within β -arrestin 1 determine the selectivity and intrinsic efficacy of downstream SH3-CP recruitment. At the second level, for which we used SRC as a model, they provided direct biophysical and cellular evidence that arrestin signals to downstream kinases using allosteric mechanisms. This study has broken the traditional idea that Arrestin only play the role of an adaptor in GPCR pathway. WANG Jiangyun (ibp) SUN Jinpeng (Shandong university) are the co-corresponding authors. YANG Fan, XIA Peng,QU Changxiu and LIU Qi from Shandong university are the co-first authors. This work was supported by the National Natural Science Foundation of China, the National Basic Research Program of China, National Science Foundation and Chinese Academy of Sciences.
Article link:
https://www.nature.com/articles/s41589-018-0085-5;
https://www.nature.com/articles/s41589-018-0115-3
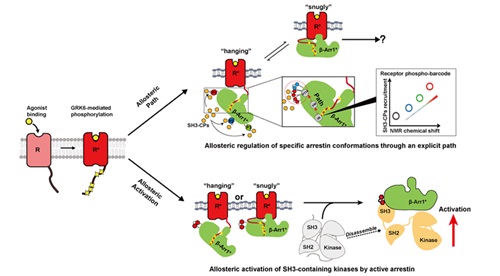
Fig 1. GPCR activates downstream signaling pathways through allosteric modulation of the complex formed by beta-arrestin-1.
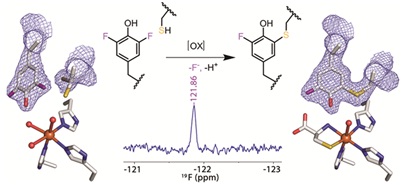
Fig 2. Crystal structure of F2-Tyr157 CDO.
Contact: WANG Jiang-Yun
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64887075
Email: jwang@ibp.ac.cn

