Atomic resolutionCryo-EM structure of Type III-ACRISPR-Cas effector complex
In bacteria and archaea, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas) proteins constitute a crRNA-guided surveillance complex that interferes with invading nucleic elements. Up to now, several CRISPR-Cas systems have been developed and applied for gene editing. The CRISPR-Cas systems can be divided into six types (Types I-VI) according to different interference mechanisms. [1, 2].Type III CRISPR-Cas system can be classified into two main subtypes: Type III-A and Type III-B. For Type III-A system, Csm (1-5) subunits assemble with crRNA to form an active effector complex, which can both degrade RNA and ssDNA. Once invading target RNA is recognized, it would be degraded by Csm3/Cmr4, meanwhile, the ssDNase of Cas10 is also activated to degrade the non-template DNA strand[3]. Recently, Type III effector complexes were demonstrated to synthesize a novel second messenger, cyclic oligonucleotide (cOA4/cOA6), for activating the nonspecific RNA degradation activity of Csm6/Csx1[4, 5]. To date, only low resolution electron microscopy (EM) structures of Csm effector complexes from different species have been reported(17-30 angstrom)[6, 7]. The precise mechanisms of the Type III-A complex assembly and action remain elusive because of the lack of the atomic resolution structure.
In a recent study from the Institute of Biophysics, Chinese Academy of Sciences, Prof. Tao Jiang and coworkers determined the atomic resolution Cryo-EM structure of Type III-A CRISPR-Cas effector complex.
In this study, the ToCsm effector complex is arranged in a ‘boot’ shape with the stoichiometry of Csm1121324151:crRNA, significantly smaller than previously reported Type III-A effectors(Fig. A). Csm1 is located at the body of the boot and interacts with Csm4 and Csm2. The C-terminus of Csm1 forms a six-helix bundle, interacting with Csm2, which mainly consists of an eight-helix bundle. Above Csm4, two Csm3 subunits (termed Csm3.1 and Csm3.2) and Csm5 are arranged along the tube of the boot, forming nearly a double-helical backbone. A 24-nt crRNA single strand was constructed running throughout the whole complex, with its 5’- end bound to Csm4 and 3’-end bound to Csm5, respectively.The thumb-like β-hairpin of Csm4, Csm3.1 and Csm3.2 induce the protrusion of nucleotides from the backbone at the 8th nt, the 14th nt and the 20th nt, resulting in 6-nt intervals in the crRNA, indicating the degraded site, which was confirmed by in vitro assay.
In addition, researchers also solved the crystal structure of ToCsm1 binding two ATP molecules at 1.69 angstrom resolution (ToCsm1crys-2ATP). The orientation of ATP molecules present in the structure is compatible with the attack of 3’-OH of the ribose of the Palm1-bound ATP on the α-P atom of the other Palm2-bound ATP molecule, presenting a pre-reaction state of the previously proposed cyclic oligonucleotide formation pocket (Fig B). The researchers constructed a well behaved C4 zinc finger motif in the ToCsm effector complex which cannot be built in ToCsm1crys-2ATP structure, showing that the C4 zinc finger may have important conformational changes to help the complex assembling (Fig C).
Moreover, previous studies showed that the HD domain activation mechanisms of Types III-A and III-B is unclear . It was predicted that a noncomplementary 3’-flank of target RNA binding channel is located between the D2 domain and the zinc finger motif of Cmr2. They found that the B domain of Csm1, corresponding to the D2 domain in Cmr2, forms a four-stranded β-sheet, which is markedly different from the D2 domain containing a four-helix bundle. By comparing with structures of PfCmr2, they found the end of the channel that is close to the 5’-handle is narrower in ToCsm effector complex than that in PfCmr complex, which may be due to the different fold of the B domain compared with the D2 of PfCmr2 that may play key roles in host self-protect and ssDNA activity activation (Fig C-D).
Taken together, this is the first atomic resolution structure of Type III-A CRISPR-Cas effector complex, which provides detailed structural basis for functional understanding. On the other hand, previous studies have shown that Type III-A CRISPR effector complex can accommodate different crRNA in length by altering the number of Csm3 and Csm2. In this structure, there are only two Csm3 and one Csm2,thus this is the smallest Csm effector complex reported to date. This may be beneficial for modifying ToCsm effector complex into an effective gene-editing tool.
This work has been published online on Cell Research on Nov. 21st, 2018, entitled “Cryo-EM structure of Type III-A CRISPR effector complex”.
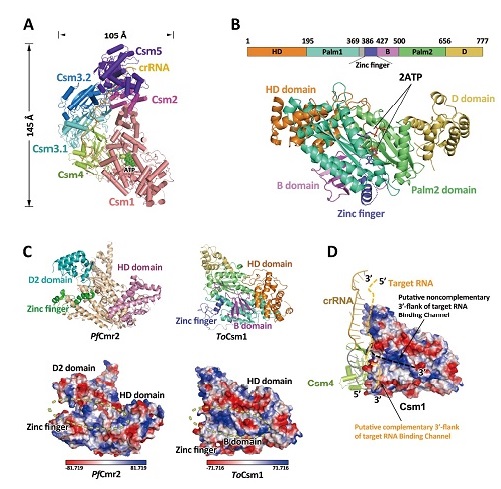
Cryo-EM structure study of Type III-A CRISPR effector complex. A. Overall Cryo-EM structure of ToCsm effector complex. B. The crystal structure of ToCsm1 with two ATP molecules. C. Comparison of zinc finger and B/D2 domain between PfCmr2 (left, PDB: 4W8Y) and ToCsm1 (right). D. Electrostatic surface potential of ToCsm1and the putative target RNA banding channel, the channel is shown by dotted line.
Article link: https://www.nature.com/articles/s41422-018-0115-6
References:
1. Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH et al: An updated evolutionary classification of CRISPR-Cas systems. Nature reviews Microbiology 2015, 13(11):722-736.
2. Tamulaitis G, Venclovas C, Siksnys V: Type III CRISPR-Cas Immunity: Major Differences Brushed Aside. Trends in microbiology 2017, 25(1):49-61.
3. Kazlauskiene M, Tamulaitis G, Kostiuk G, Venclovas C, Siksnys V: Spatiotemporal Control of Type III-A CRISPR-Cas Immunity: Coupling DNA Degradation with the Target RNA Recognition. Molecular cell 2016, 62(2):295-306.
4. Niewoehner O, Garcia-Doval C, Rostol JT, Berk C, Schwede F, Bigler L, Hall J, Marraffini LA, Jinek M: Type III CRISPR-Cas systems produce cyclic oligoadenylate second messengers. Nature 2017, 548(7669):543-548.
5. Kazlauskiene M, Kostiuk G, Venclovas ?, Tamulaitis G, Siksnys V: A cyclic oligonucleotide signaling pathway in type III CRISPR-Cas systems.pdf. Science 2017, 357:605–609.
6. Staals RH, Zhu Y, Taylor DW, Kornfeld JE, Sharma K, Barendregt A, Koehorst JJ, Vlot M, Neupane N, Varossieau K et al: RNA targeting by the type III-A CRISPR-Cas Csm complex of Thermus thermophilus. Molecular cell 2014, 56(4):518-530.
7. Rouillon C, Zhou M, Zhang J, Politis A, Beilsten-Edmands V, Cannone G, Graham S, Robinson CV, Spagnolo L, White MF: Structure of the CRISPR interference complex CSM reveals key similarities with cascade. Molecular cell 2013, 52(1):124-134.
Contact: Tao Jiang
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101,China
Phone: 86-10-64888510
Email: tjiang@ibp.ac.cn
(Reported by Jiang Tao's group)

