Structural Insights into the Non-canonical Ubiquitination Catalyzed by SidE
Ubiquitination is one of the most abundant post-translational modifications that influences a broad spectrum of cellular functions in eukaryotes. Conventional ubiquitination requires ATP and is achieved by the concerted action of the activating (E1), conjugating (E2), and ligating (E3) enzymes, ultimately resulting in an isopeptide bond between the C-terminus of ubiquitin and an acceptor lysine residue of the substrate protein.
Given the importance of ubiquitination in many cellular processes including the regulation of the immune system, it is not surprising that the ubiquitination network is exploited by a variety of microbial pathogens. The newly identified SidE family effectors of the pathogen Legionella pneumophila ubiquitinate several human proteins by a novel mechanism without engaging any of the conventional ubiquitination machinery. The SidE family includes four large proteins (SidE, SdeA, SdeB, and SdeC). Starting from the N-terminus, SidE family proteins consist of four domains: a DUB domain, a phosphodiesterase (PDE) domain, a mono-ADP-ribosyltransferase (mART) domain, and a coiled-coil (CC) domain. The mART domain of the SidE family can covalently attach the ADP-ribose moiety of the cofactor NAD to the R42 residue of ubiquitin to form the activated ADP-ribosylated ubiquitin (ADPr-Ub) intermediate. This intermediate is subsequently catalyzed by the PDE domain to release AMP and phospho-ribosylated ubiquitin (Pr-Ub), which is the ubiquitin moiety that is ultimately attached to the serine residue of substrate proteins through a phosphor-ribosyl linkage. However, the molecular mechanisms of this non-canonical ubiquitination machinery are still unclear.
In the research paper entitled ”Structural Insights into the Non-canonical Ubiquitination Catalyzed by SidE” published by Cell on May 3, 2018, Prof. GAO Pu from Institute of Biophysics reported several high-resolution structures of SidE bound to ubiquitin and cofactors, revealing unexpected features of this all-in-one ubiquitination machinery.
Based on these structures, researchers found that: 1). The catalytic pockets of the mART and PDE domains are far from each other and face in different directions, suggesting that the two catalytic steps of non-canonical ubiquitination are likely independent of each other. Meanwhile, the PDE-mART dual domain forms a rigid inter-domain conformation, which maintains high activity of SidE family; 2). Ubiquitin is attached to one end of the NAD-binding cleft of the mART domain. The interaction surface in the mART domain comprises two small acidic pockets, which were filled by the two arginine residues (R72 and R74) of ubiquitin. Most of the intermolecular interactions were mediated by the C-terminal tail of ubiquitin. NAD binds in the deep cleft of the mART domain, which contains a conserved R-S-E motif, and the nicotinamide ribose moiety of NAD is close to the bound ubiquitin; 3). Most of the intermolecular interactions were mediated between the K6-T9 fragment and His68 of ubiquitin and the PDE domain. R42 side chain of ubiquitin points into the groove. Upon binding to ubiquitin, PDE undergoes local conformational changes to form interactions with ubiquitin; 4). SidE proteins do not recognize specific structural folds of substrates, but rather can modify a much larger substrate pool as long as the serine residue of the substrate protein can bind into the catalytic pocket of the PDE domain without the steric effect. In summary, this work provides detailed information to explain the molecular mechanism of the non-canonical ubiquitination catalyzed by SidE family enzymes. This work will also serve as an important reference in designing novel tools with potentially far-reaching implications.
This work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences Pilot Strategic Science and Technology Projects B, the Starting-Up Funding of the Institute of Biophysics.
Article link:https://doi.org/10.1016/j.cell.2018.04.023
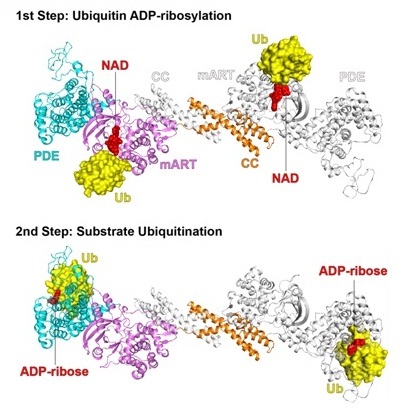
Structures of mART-ubiquitin-NAD and PDE-ubiquitin-ADPr
Contact: GAO Pu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64887199
Email: gaopu@ibp.ac.cn

