Near-atomic Structure of Japanese Encephalitis Virus Reveals Critical Determinants of Virulence and Stability
Using a combination of structural analysis, cellular assays, reverse genetics and animal studies using mouse models, an international group of scientists have clarified the molecular mechanisms for neurovirulence and viral stability, which extends our understandings of the serocomplex-specific pathogenesis of encephalitic flaviviruses.
Flaviviruses, including West Nile virus (WNV), Japanese encephalitis virus (JEV), dengue virus (DENV), yellow fever virus (YFV) and Zika virus (ZIKV), are positive-stranded RNA viruses group that are mainly transmitted through mosquitos and ticks. These viruses can cause serious human diseases. JEV, WNV, St. Louis encephalitis virus (SLEV) and Murray Valley encephalitis virus (MVEV), belonging to the JEV group, have been consistently shown to be associated with human cases of encephalitis. Worldwide, approximately 50,000-175,000 clinical cases of Japanese Encephalitis are reported in more than 25 countries annually
Professor RAO Zihe and Professor WANG Xiangxi at the Institute of Biophysics (IBP) of the Chinese Academy of Sciences, in cooperation with Professor QIN Cheng-Feng at the Beijing Institute of Microbiology and Epidemiology and Professor David Stuart in Oxford University and colleagues applied cryo-electron microscopy to determine the cryo-EM structure of mature JEV at near-atomic resolution. The high resolution structure of JEV reveals an unusual “hole” on the surface, surrounded by five encephalitic-specific motifs implicated in receptor binding. Glu138 of E, which is highly conserved in encephalitic flaviviruses, maps onto one of these motifs and is essential for binding to neuroblastoma cells, with the E138K mutation abrogating neurovirulence and neuroinvasiveness of JEV in mice. In additions, researchers have identified structural elements modulating viral stability, notably Gln264 of E, which, where replaced by His264 strengthens a hydrogen-bonding network, leading to a more stable virus, which is attenuated.
The research work entitled Near-atomic structure of Japanese encephalitis virus reveals critical determinants of virulence and stability was published on line in the journal of Nature Communications on April 26, 2017. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the Ministry of Science and Technology 973 Project, the Newton Advanced Fellowship from the UK Academy of Medical Sciences and NSFC and National Science Foundation.
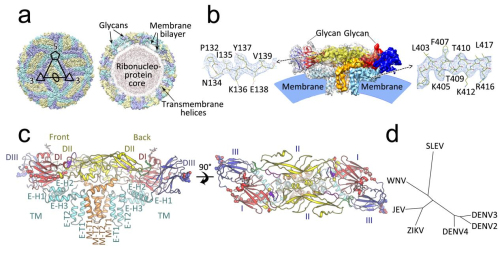
Fig.1 Overview of the cryo-EM structure of the JEV (Image by IBP)
Contact:
RAO Zihe
National Laboratory of Biomacromolecules, IBP
E-mail:raozh@sun5.ibp.ac.cn
Tel:010-64888556

