The group of Prof. Sarah Perrett has made important progress in the study of mechanisms of amyloid fibril formation
The self-assembly of polypeptides into amyloid structures is associated with a range of increasingly prevalent neurodegenerative diseases as well as with a select set of functional processes in biology. The phenomenon of self-assembly results in species with dramatically different sizes, from small oligomers to large fibrils; however, the kinetic relationship between these species is challenging to characterize by traditional ensemble methods.
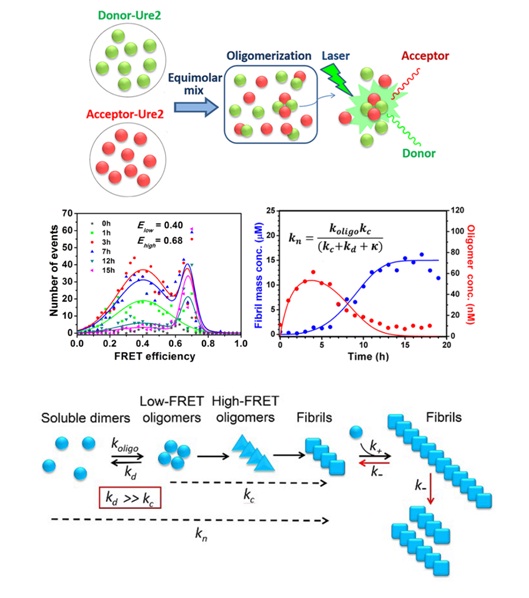
In this study, we used single molecule fluorescence resonance energy transfer (smFRET) to obtain quantitative information on the oligomer populations formed during aggregation of the yeast prion protein Ure2. We identify an initial metastable oligomer, which can subsequently convert into a structurally distinct oligomer, which in turn converts into growing fibrils. In collaboration with Prof. Tuomas Knowles from the Department of Chemistry, University of Cambridge, a multi-step kinetic model was produced and used to globally analyze the smFRET and bulk ThT data, revealing the molecular mechanism underlying oligomer formation and depletion. We also show that fragmentation is responsible for the autocatalytic self-replication of Ure2 fibrils, in contrast to the mechanism observed for peptides and proteins associated with neurodegenerative disease, which generate amyloid nuclei predominantly through secondary nucleation. This study establishes a framework for elucidating the temporal and causal relationship between oligomers and larger fibrillar species in amyloid forming systems, and provides insights into why functional amyloid systems are not toxic to their host organisms.
This study entitled “Direct Observation of Oligomerization by Single Molecule Fluorescence Reveals a Multistep Aggregation Mechanism for the Yeast Prion Protein Ure2” was publishedon-line in the Journal of the American Chemical Society on January 22, 2018.Prof. Sarah Perrett and Dr. Si Wu, both from the Institute of Biophysics, CAS, and Prof. Tuomas Knowlesfrom the University of Cambridge are the corresponding authors.Jie Yang, a PhD student in Prof. Sarah Perrett’s group, and Alexander Dear, a PhD student in Prof. Tuomas Knowles‘s group, are the co-first authors. The work was funded by the National Natural Science Foundation of China and the Ministry of Science and Technology of China.
Article link: http://pubs.acs.org/doi/10.1021/jacs.7b10439
CONTACT:
Sarah Perrett, Si Wu
Institute of Biophysics, Chinese Academy of Sciences
Beijing 100101, China
Phone: 86-10-64889870
Email: sperrett@ibp.ac.cn
wusi@moon.ibp.ac.cn

