Thymic portal endothelial cells was revealed
Trafficking of hematopoietic cells to and from lymphoid tissues is a basic biological process underling many important immunological functions under both physiological and pathological conditions. Exploring the cellular basis and understanding the molecular mechanisms have been of great interest. While the knowledge of cell trafficking to secondary lymphoid tissues has been steadily advanced, especially since the discovery of high endothelial cells by Dr. Eugene Butcher in 1988, the specialized endothelial cells responsible for thymic homing still remain a longstanding mystery, even after decades of efforts. Therefore, the nature of thymic cell entry remains poorly understood.
In a recent study published in Nature Communications, a group led by Dr. ZHU Mingzhao from Institute of Biophysics, Chinese Academy of Sciences, reported that lymphotoxin beta receptor (LTbR) controlled a specialized population of thymic endothelial cells (thymic portal endothelial cells) for thymic progenitor cell entry. This population of endothelial cells are predominantly located with perivascular spaces and serve as the gate for thymic progenitor cell homing. RNA-seq analysis revealed obvious molecular signatures of TPECs related to cell adhesion and migration. LTbR plays an essential role for the differentiation and homeostasis of TPECs. Interestingly, the positively selected mature T cells were found to directly control these TPECs for progenitor cell homing. Functionally, LTbR deficiency resulted in dramatic defect of thymic progenitor cell homing and impaired thymic regeneration upon injury. Thus, for the first time, the study has identified the key portal endothelial cells in the thymus for progenitor cell homing and suggests an unrecognized feedback regulation of thymic progenitor cell homing by the mature T cells in an LTbR dependent manner.
Given the increasingly acknowledged importance of thymic homing of various hematopoietic cells (including dendritic cells, Treg cells and B cells) for T cell development and central tolerance, the current finding of TPECs will not only open a new avenue for the research on thymic trafficking, but also provide novel insights for further understanding and manipulation of T cell development and central tolerance.
The study was funded by Ministry of Science and Technology of China, National Natural Science Foundation of China and Chinese Academy of Sciences.
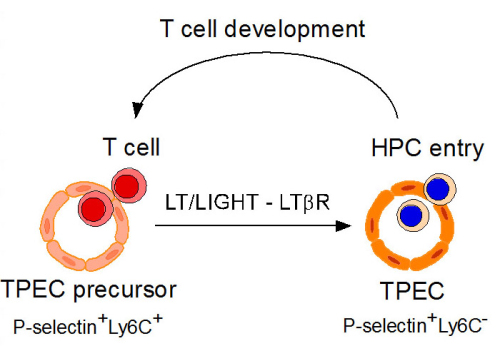
T Cell Development (Image by IBP)
Contact
ZHU Mingzhao
Chinese Academy of Sciences Key Laboratory of Infection and Immunity,IBP
E-mail:zhumz@ibp.ac.cn,Tel:010-64888775

