Structural study of MCPIP1 N-terminal conserved domain reveals a PIN-like RNase
The immune system is the natural barrier that protects our body and fights against harmful germs. Immune regulation is the ability of the immune system to control and regulate its own responses to make sure it fights against invading organisms but does not overreact to neutral or friendly organisms. Mistakes and dysfunction in immune regulation may lead to diseases such as cancer, diabetes, allergies, and rheumatic arthritis. The aim of studies on immune regulation is to understand the underlying mechanisms and develop biomedical methods to prevent and treat immunological diseases.
Many of the scientists at the Institute of Biophysics, Chinese Academy of Sciences are focusing on immune regulation from different perspectives. Academician RAO Zihe and Professors LI Xuemei and GAO Guangxia have been working together to solve the mystery of MCPIP1, a protein that plays an important role in immune regulation.
Previous studies have shown that MCPIP1 knockout mice have a very short lifespan of less than 12 weeks and suffer from severe immune disorders. The substrates of MCPIP1 include certain pre-miRNAs and mRNAs of inflammatory cytokines such as IL-6, and IL-12p40, all of which are important regulators in many immune responses. As a result, the RNase activity of MCPIP1 has received a lot of attention.
Recently, Professor RAO and his colleagues reported the first 3D structure of the MCPIP1 N-terminal conserved domain in the journal Nucleic Acids Research. They found that although the N-terminal conserved domain of MCPIP1 shares only remote sequence homology with the PilT N-terminal domain, they share high structural similarity, confirming that the N-terminal conserved domain is a PIN-like RNase. They also identified the catalytic center of MCPIP1 using magnesium soaking methods. Four aspartic acid residues are crucial for the RNase activity of MCPIP1, as they are needed for stabilizing either the magnesium ion or the water molecules surrounding the magnesium ion. Single mutation of any of these four aspartic acids residues results in a complete loss of RNase activity.
IBP researchers also found that a positively charged arm near the catalytic center may play important roles in substrate binding. Mutation of the positively charged residues results in loss of RNase activity. In vitro activity experiments also suggested that the N-terminal conserved domain alone is sufficient for RNA degradation. However, both the N-terminus and the zinc finger motif are needed for efficient RNA degradation by MCPIP1.
This study provides new insights into the RNase activity of MCPIP1 which are very important for further understanding the immune regulatory role of MCPIP1. This work was supported by grants from the Ministry of Science and Technology of China, Natural Science Foundation of China and the Chinese Academy of Sciences.
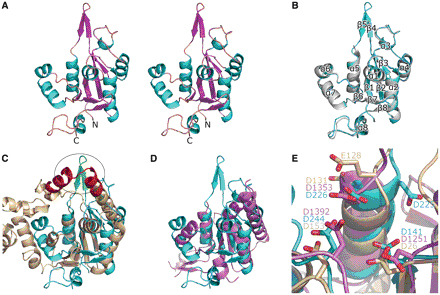
Figure 1. Overall structure of MCPIP1 NCD and its comparison with homologous proteins. (A) Stereo-view of the crystal structure of MCPIP1NCD-ZF. The structure of MCPIP1 NCD-ZF adopts an a-b-a sandwich-like fold. The residues 112–223 and the zinc-finger motif (residues 297–334) are not included in the model due to absence of interpretable electron density. (B) Superposition of the NCD-ZF structure on the NCD structure. The NCD structure is in violet and NCD-ZF in cyan. The eight a-helices and eight b-strands in the structure are numbered from the N-terminal to the C-terminal respectively. There are only slight differences in the loop region between these two structures. (C) Superposition of the MCPIP1 NCD-ZF structure and the T5 D15 50-exonuclease structure (pdb code: 1exn). The 1exn structure is colored in wheat and NCD-ZF in cyan. The oval circle indicates the positively charged arm of MCPIP1 (green) in the same position as the nuclear acid-binding arch of 1exn (red). (D) Superposition of the MCPIP1 NCD-ZF structure and the PIN domain of human SMG6 (pdb code: 2hww). The 2hww is colored in violet. A good fit in the central b-sheets and several a-helixes can be seen in this figure. (E) Conserved residues and their similar arrangement in the catalytic centers of MCPIP1NCD-ZF (cyan), T5 D15 50-exonuclease (wheat) and the PIN domain of human SMG6 (violet). Residues involved in the catalytic center are shown in stick representation and labeled. (From RAO Zihe, LI Xuemei and GAO Guangxia )

