Novel epigenetic mechanism of Notch signaling regulation during Drosophila development revealed
The Notch pathway is an evolutionarily conserved signaling cascade that plays essential roles in the control of cell proliferation and cell fate specification during animal development. Defects in the Notch signaling are associated with various types of human disorders, such as cancer.
In the last few decades, scientists have been working to understand how this signaling process is regulated, and large progress has been made. However, how this pathway is regulated at the chromatin level is not fully understood. Thus, investigation of Notch signal transduction at this level should shed more light on understanding how this pathway is regulated and may provide more cues for intervening related diseases
In fruit flies, chromatin assembly factor 1 (CAF-1) is a three subunits (p180, p105 and p55) protein complex, which was first identified as a replication-coupled histone chaperone. Previous studies of CAF-1 have been focused on three aspects: replication-coupled chromatin assembly, local chromatin re-establishment after DNA repair and heterochromatin duplication. However, loss of function phenotypes in animals suggest that CAF-1 may have a role in regulating multicellular animal development, which remains poorly defined thus far.
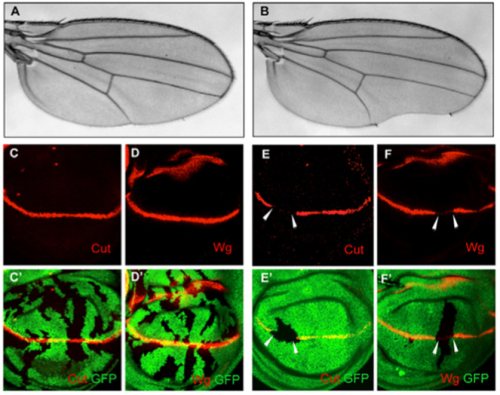
Through years of constant studies, for the first time, Dr. JIAO Renjie and his group at the Institute of Biophysics, Chinese Academy of Sciences discovered the function of CAF-1 in Notch signaling during the fruit fly development. They found that disruption of the middle subunit of CAF-1, dCAF-1-p105, leads to a phenotype that resembles the Notch loss of function phenotype (Figure 1B), and affects cell autonomously the Notch pathway target genes’ expression (Figure 1E, E’, F and F’, white arrows). At the molecular level, dCAF-1-p105 is associated with the Notch pathway core transcriptional factor Su(H) to regulate the enrichment of acetylated H4 (H4ac) at the enhancer regions of Notch targets. Their work indicates that chromatin assembly factors may function as a co-activator of Notch signaling transduction during normal animal development.
Figure 1. dCAF-1-p105 loss of function leads to Notch wing phenotypes and down-regulated Cut and Wg expression
This work has been recently published in the journal of Development (http://dev.biologists.org/content/140/17/3635), and was supported by the 973 program and National Natural Science Foundation of China.