New mechanism of Pcid2 in regulating pluripotency of embryonic stem cells revealed
Embryonic stem cells (ESCs) are pluripotent. They have capability to self-renew in vivo and in vitro through expression of pluripotency genes and down-regulation of commitment genes. ESCs become specialized cell types during differentiation through activation of the specific transcriptional factors, up-regulation of lineage-specific genes and repression of the pluripotency genes. It has not been well characterized about regulation of the fate switch between self-renewal and differentiation of ESCs.
A recent study reveals a new mechanism of the switch balance between maintenance of ESC pluripotency and differentiation. This study was lead by Professor FAN Zusen, an immunologist from the Institute of Biophysics, Chinese Academy of Sciences.
Professor FAN’s group studied the complicated epigenetic controlling mechanisms in regulating gene expression in embryonic development and in stem cell differentiation. They targeted at the CBP (CREB-binding protein)/p300 family, a histone acetyltransferase (HAT) and a transcriptional co-activator that plays pivotal roles in embryogenesis, transcription regulation and ESC differentiation. They found that Pcid2 (PCI-domain containing protein), a homolog of the yeast Thp1, is present in the CBP/p300-EID1 complex. Both Pcid2 knockdown and Pcid2 deficiency impair the pluripotency of mESCs and hESCs in vitro, decline the expression level of AP and Oct4 in ESCs. They demonstrated that Pcid2 depletion resulted in failure of the 3 embryonic layer generation via an in vivo teratoma formation assay. They discovered that EID1 interact with Pcid2 using a yeast two-hybrid approach. Pcid2 associates with EID1 and is present in the CBP/p300-EID1 complex of ESCs. Overexpression of Pcid2 could disrupt the interaction of MDM2 with EID1 in mESCs indicating that Pcid2 and MDM2 competitively bind to EID1. Pcid2 depletion remarkably increased CBP/p300 HAT activity resulting in enhanced histone 3 acetylation in the promoters of developmental genes but not the pluripotency genes. Overexpression of the stable mutant of EID1 and using the MDM2 inhibitor caused the rescue of stemness in Pcid2 silenced mESCs.
This study provides new insights into the molecular mechanism of Pcid2 in the regulation of switch balance between maintenance of pluripotency and differentiation in mouse and human embryonic stem cells. This discovery sheds new light in developing novel immunotherapies for diseases such as cancer.
The researchers published their results in the Stem Cells journal. The article entitled “Pcid2 Inactivates Developmental Genes in Human and Mouse Embryonic Stem Cells to Sustain Their Pluripotency by Modulation of EID1 Stability” was co-first authored by Drs. Buqing Ye and Zhonghua Dai.
The work was supported by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Sciences and the China Postdoctoral Science Foundation.
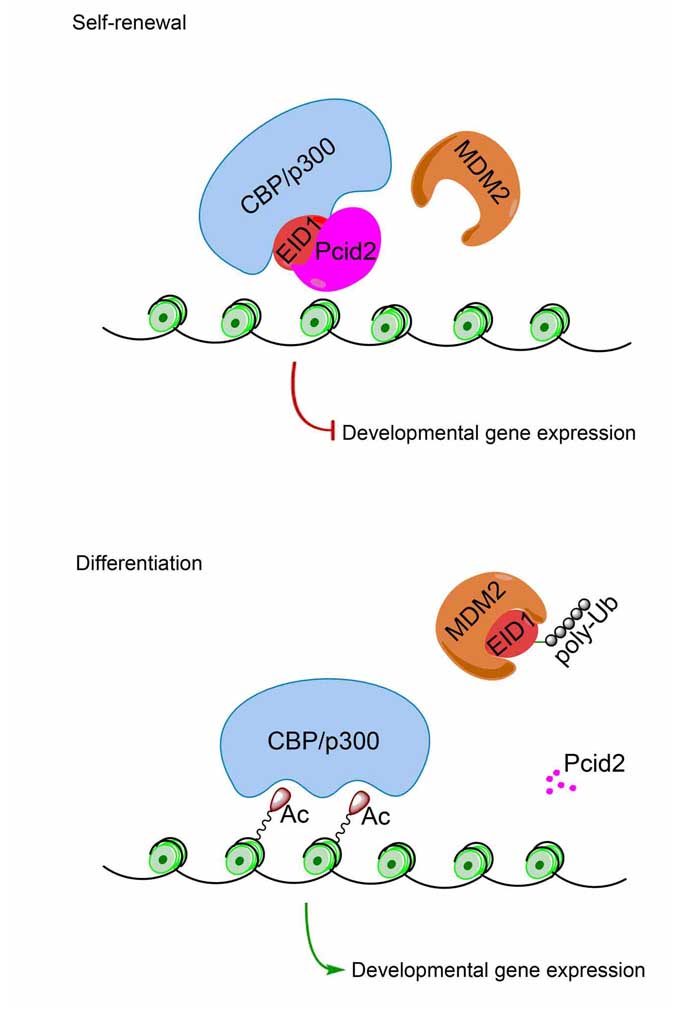
Figure. Pcid2 regulates pluripotency maintenance of embryonic stem cells.

