New insights into the causes of mitochondrial catastrophe
Mitochondrial catastrophe can be the cause or consequence of apoptosis and is associated with a number of pathophysiological conditions such as diabetes, neurodegenerative diseases and cancers. In a collaborative study between Prof. XU Jianxin’s lab at the Institute of Biophysics, Chinese Academy of Sciences and Prof. CHEN Quan of the Joint Laboratory of Apoptosis and Cancer Biology at the National Key Laboratory of Biomembrane and Membrane Biotechnology (CAS) and the College of Life Sciences at Nankai University, recently reported in the journal Cell Research, Zhu et al. investigate the relationship between mitochondrial catastrophe and caspase activation, addressing the underlying mechanism of caspase activation and explaining how activated caspase can feedback to attack mitochondria, further amplifying cytochrome c release.
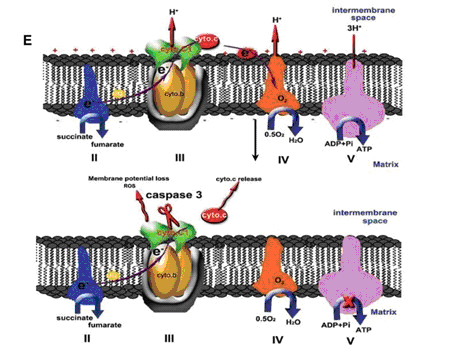
Zhu et al. first show that cytochrome c1 of the mitochondrial respiration chain bc1 complex is a substrate of caspase 3. They demonstrate that cleavage of cytochrome c1 causes mitochondrial catastrophe, manifesting in impaired mitochondrial ATP generation, increased ROS and profound mitochondrial fragmentation. They then go on to show that caspase 3 cleaves cytochrome c1 at the D106 site that is critical for binding with cytochrome c following apoptotic stresses or targeted expression of caspase 3 in the mitochondrial intermembrane space. This cleavage may disrupt the interaction of cytochrome c1 with the bc1 complex and electron transfer between the bc1 complex and complex IV, resulting in further amplification of mitochondrial cytochrome c release and subsequent metabolic catastrophe. Mutagenesis and inhibitor studies then showed that these mitochondrial events can be blocked effectively by expressing non-cleavable cytochrome c1 (a D106A mutant) or by the caspase inhibitor z-VAD-fmk.
Professors CHEN, XU and colleagues conclude that cleavage of cytochrome c1 represents a critical step for the feedback amplification of cytochrome c release by caspases and subsequent mitochondrial catastrophe, and that mitochondrial dysfunction induced by caspase feedback attack is important for the progression of apoptosis. Their results offer a fresh understanding of how metabolic catastrophe is causally linked with apoptosis and provide a better understanding of the interplay of these complex processes which may be useful in the fight against metabolic and aging-related diseases.
Cell Research (2012) 22:127-141. doi:10.1038/cr.2011.82
 |
|
Pig. Model of how cyto.c1 cleavage by casp.3 affects cyto.c release. The cytochrome bc1 complex is the centralcomponent of the oxidative respiratory reaction chain that oxidizes ubiquinol and reduces cyto.c. Casp.3 cleavage of cyto.c1disrupts the electron transport chain leading to an increase in ROS, ample release of cyto.c, the loss of mitochondrial membranepotential and mitochondrial function for ATP generation (lower). (From XU Jianxing et.al)

