New findings on the mechanism by which granzyme M causes caspase activation leading to the lysis of intracellular pathogens and tumor cells
Intracellular pathogens and tumor cells are targeted and killed by cytotoxic lymphocytes such as natural killer (NK) and cytotoxic T-lymphocyte (CTL) cells.
The Granzyme (Gzm)/perforin pathway, one of the major pathways which lead to such cytolysis, involves Gzms, serine proteases that induce distinct cell death pathways by proteolysing different target cell substrates, and perforin, a protein that assists the entry of Gzms into the target cell cytosol.
Granzyme M (GzmM), a chymotrypsin-like serine protease that preferentially degrades its substrates after Met or Leu residues, is constitutively and abundantly expressed in the innate effector NK cells which play an essential role in eradicating tumors at early stages.
In a paper published recently in Cell Death and Differentiation1, Prof. FAN Zusen’s lab at the Institute of Biophysics, Chinese Academy of Sciences investigate the role of GzmM in NK cell-mediated cytolysis, elucidating the mechanism by which it induces caspase (casp)-dependent apoptosis and cytochrome c release from mitochondria.
Wang et al. found that casp-8 is the initiator caspase in the GzmM-induced casp cascade which causes activation of other caspases and the cleavage of Bid, demonstrating that casp-8 knockdown or deficiency attenuates or abolishes GzmM-induced proteolysis of procaspase-3 and Bid.
They show that FADD (Fas-associated protein with death domain), an extrinsic death receptor pathway adaptor, contributes to GzmM-induced casp-8 activation. GzmM specifically cleaves FADD after Met 196 to generate truncated FADD (tFADD), enhancing its self-association and promoting its oligomerization. Oligomerization of tFADD then facilitates procaspase-8 recruitment to promote its auto-processing leading to the casp activation cascade. FADD-deficient cells abrogate GzmM-induced activation of casp-8 and apoptosis as well as significantly inhibiting lymphokine-activated killer cell-mediated cytotoxicity.
These findings provide new insights into the apoptosis pathway induced by NK cells, and demonstrate that FADD processing by GzmM potentiates the killing efficacy of NK cells against tumor cells and intracellular pathogens. This study was funded by the National Natural Science Foundation of China, the 973 Program, and the Innovation Program of the CAS.
1. S. Wang, P. Xia, L. Shi and Z. Fan: FADD cleavage by NK cell granzyme M enhances its self-association to facilitate procaspase-8 recruitment for auto-processing leading to caspase cascade. Cell Death and Differentiation (2012) 19, 605–615.
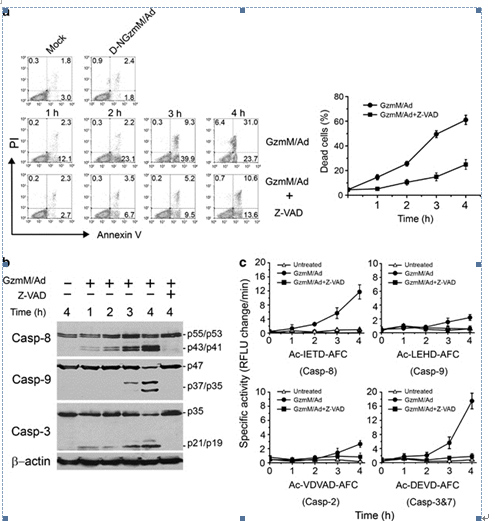
Figure 1. Casp activation cascade is required for GzmM-induced apoptosis. (a) Casp pan inhibitor Z-VAD can restrain GzmM-induced apoptosis. Jurkat cells (2 × 105) were pretreated with 100?μM Z-VAD for 30?min before 1?μM GzmM plus Ad treatment at the indicated time points (37°C). Cells were stained with FITC-conjugated Annexin V and PI for flow cytometry. Cells treated with enzymatically inactive mutant D-NGzmM/Ad for 4?h served as a negative control. Total dead cells were calculated by Annexin V and PI single-positive as well as Annexin V/PI double-positive cells and are shown as means ± S.D. in the right panel. These data are representative of three independent experiments. (b) GzmM-induced casp cascade is blocked by Z-VAD. Treated Jurkat (2 × 105) cells as above were probed by specific antibodies against casp-8, -9 and -3. The same blot was stripped for immunoblotting. β-actin was detected as a loading control. (c) Casp activations are confirmed by casp-specific fluorogenic substrates. The cell lysates from the above treated Jurkat cells were incubated with Ac-IETD-AFC, Ac-LEHD-AFC, Ac-VDVAD-AFC or Ac-DEVD-AFC for assessment of casp-8, -9, -2 and -3/7 activity, respectively. Casp activity is shown as relative fluorescent light units (RFLU) change per minute. (From FAN Zusen et. al)

