Structural study of norovirus-Lewis antigen interaction indicates its type-specific HBGA recognition
Noroviruses are a group of small-round structured, non-enveloped RNA viruses causing epidemic acute gastroenteritis in both developing and developed countries. Norovirus possesses an outer protein capsid that encapsulates a single-stranded, positive sense RNA genome. The capsid can be divided into the N-terminal shell (S) and the C-terminal protruding (P) domains, both found to be structurally and functionally independent. The S domain forms the interior shell while the P domain constitutes the arch-like P dimer of the capsid. Functional analyses showed that the P domains could form stable dimers which bind to histo-blood group antigens (HBGAs), the host susceptible factors of noroviruses. The HBGA binding is a prerequisite of norovirus infection and therefore important in the evolution of human noroviruses.
Professor Zihe Rao’s research team at the Institute of Biophysics, Chinese Academy of Sciences recently determined the crystal structures of the P domain protein of the first Lewis-binding norovirus (VA207, GII.9) and revealed the mechanism of its binding specificity in collaboration with Professor Xi Jiang’s lab at Division of Infectious Diseases, Cincinnati Children's Hospital Medical Center. Co-crystallization of the VA207 P dimer with Ley and sialyl Lex tetrasaccharides showed that VA207 interacts with these antigens through a common site which is highly conserved among most GII noroviruses. However, the HBGA-binding site of VA207 targeted at the Lewis antigens through the α-1, 3 fucose (the Lewis epitope) as major and the β-N-acetyl glucosamine of the precursor as minor interacting sites. These data for the first time elucidate the genetic and structural basis of strain-specificity by a direct comparison of two genetically related noroviruses in their interaction with different HBGAs, highlighting the role of human HBGA as a critical factor in norovirus evolution. The structural data would help antiviral development for disease control and prevention of noroviruses.
This work was published in the latest issue of PLoS Pathogens on July 21, 2011 (2011, 7(7): e1002152) and titled “Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens”.
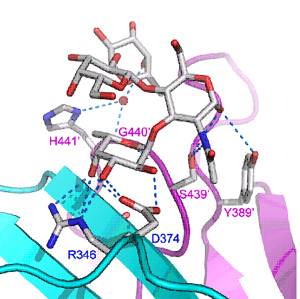 |
Fig. The crystal structure of the HBGA-binding interface of norovirus VA207, showing the extensive interaction network with Lewis Y tetrasaccharide. by Zihe Rao et al.

