New Findings on the Folding and Folding Stability of Staphylococcal Nuclease
Professor Jinfeng Wang’s group have a special interest in studying the folding mechanism of Staphylococcal nuclease (SNase) in vitro, investigating the tertiary interactions that are necessary for the folding of SNase fragments into native-like conformation, the interactions between SNase N- and C-terminal subdomains, the relationship between enzyme activity and the folding and dynamic states of SNase, and the structural properties of the enzyme protein that are responsible for its function.
SNase is a widely used model system for the study of protein folding and is composed of two subdomains, a large N-terminal β-subdomain and a small C-terminal a-subdomain. The N-terminal β-subdomain contains a “β-barrel” core, two helices (a1: A58-A69; a2: V99-Q106), a p-loop (pdTp-binding loop, D77-L89) and a Ω-loop (P42-P56), while the C-terminal a-subdomain contains a helix (a3: E122-K136) and two loops, a N-terminal loop (V111-H121) and a C-terminal loop (L137-Q149) which contains a helical turn (L137-S141).
In a recent paper in Biochemistry1, Prof Wang’s group reported new findings on the role of the C-terminal loop (L137-S141) of SNase in its folding and stability. They analysed the folding states of C-terminal truncated SNase fragments, SNase137, SNase139, SNase140, and SNase141 containing residues 1-137, 1-139, 1-140, and 1-141, respectively, using circular dichroism and fluorescence measurements, and used heteronuclear NMR to determine the solution structure of SNase140 and compare it with those of SNase141 and native SNase. Results indicated that folding of the four SNase fragments was correlated with the folding of helix a3. As chain length extended from L137 and I139 through to S141, folding of the fragments progressively approached the tertiary folding of native SNase, and folding stability was enhanced. They conclude that the C-terminal loop L137-S141 has a profound effect not only on the folding of helix a3 but also on the folding stability of both the a- and β-subdomains of SNase. Folding stability of the SNase and SNase fragments depends to a large extent on the hydrophobic packing interactions in both the C-terminal local structural region surrounding the W140 residue, including the L137-S141 loop, and the N-terminal local structural region of the “β-barrel” hydrophobic core.
1. Min Wang, Yingang Feng, Hongwei Yao, and Jinfeng Wang (2010): Importance of the C-Terminal Loop L137-S141 for the Folding and Folding Stability of Staphylococcal Nuclease Biochemistry 49: 4318–4326
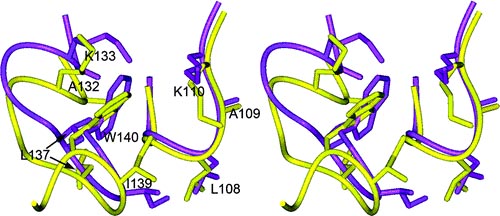
View of the side chain packing of the pyrrole ring of W140 in the hydrophobic environment formed by side chains of residues G107, L108, A109, A132, L137, and I139 and the long hydrophobic side chains (Cβ?ζ) of amphiphilic residues K110 and K133 in SNase140 (purple) and native SNase

