IBP – an improved method for the detection of S-nitrosation in proteins
Professor Chang Chen’s laboratory is interested in the mechanism and cellular effects of redox-based post-translational modifications of proteins, particularly the S-nitrosation of proteins induced by reactive nitrogen species, including nitric oxide and its derivatives.
Over 95% proteins are thought to contain cysteine, a nucleophilic amino acid whose modification by various electrophiles participates in the regulation of protein function. Post-translational modifications of proteins involving cysteine are known to be important regulatory mechanisms for signal transduction. However, methods for precisely discriminating between the various redox-related cysteine modifications (including protein disulfide bond (-S-S-), S-nitrosothiol (-SNO), sulfenic acid (-SOH), sulfinic acid (-SO2H), and sulfonic acid (-SO3H)) are limited. As a result the study of the relationship between cysteine modifications and protein function has been hampered.
The invention of the biotin switch assay in 2001 for detecting S-nitrosation was a significant breakthrough, and this assay has since been widely used in the field of nitric oxide and redox signaling to isolate the biological effects of S-nitrosation from other cysteine modifications. As a result, hundreds of targets have been identified and the role of S-nitrosation in cellular signaling and diseases has been explored. However, while using the biotin switch assay routinely in their work, Professor Chen’s lab discovered that intermolecular disulfide bonds interfere with the accuracy of the identification of S-nitrosated proteins in this assay; proteins which are linked to S-nitrosated proteins by intermolecular disulfide bonds can be falsely detected as S-nitrosated targets. They then made some innovative improvements to the assay and these have recently been published in the journal Free Radical Biology and Medicine1. Huang et al’s new method, called “Irreversible Biotinylation Procedures”, or IBP for short, improves the accuracy of the assay by preventing interference from intermolecular disulfide bonds using irreversible biotinylation instead of reversible biotinylation and breaking all the intermolecular disulfide bonds in the sample before biotinylated proteins are purified. This novel strategy allows specific detection of protein S-nitrosation without the potential interference of intermolecular disulfide bonds. Huang et al also developed proteomic approaches and quantitative proteomic approaches for high-throughput studies and quantitation of protein S-nitrosation which can be used alongside the new procedures.
Professor Chen’s work will no doubt lead to the re-evaluation of the influence of intermolecular disulfide bonds on previously reported results based on the original biotin switch assay. It is possible that cellular processes previously thought to be related to S-nitrosation can now only be said to be redox-related cysteine modifications. This work thus raises important questions for the field of redox signaling.
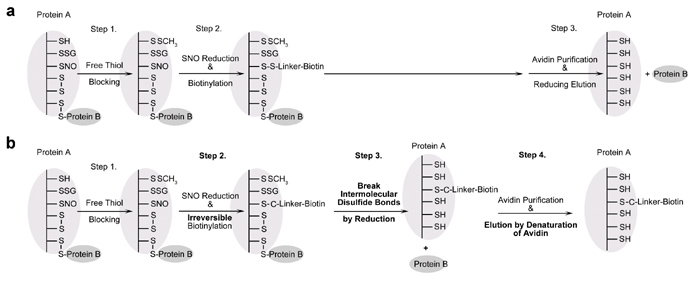
Figure 3. Theoretical analysis of the original biotin switch assay followed by avidin purification, and the construction of the irreversible biotinylation procedure, “IBP”. a. Flow chart for the original biotin switch assay. Protein B will be falsely detected as a protein target of S-nitrosation because the intermolecular disulfide bond is not broken before purification. b. Flow chart for IBP, which eliminates the interference from protein B by adding a reduction step to release protein B before purification.
1. Bo Huang, Chang Chen (2010) Detection of Protein S-Nitrosation using Irreversible Biotinylation Procedures (IBP). Free Radical Biology and Medicine 49: 447-457

